All published articles of this journal are available on ScienceDirect.
Prevalence of Selected Risk Factors for Cardiometabolic Disease among University Staff in the Western Cape, South Africa
Abstract
Introduction:
This study aimed to determine the prevalence of selected risk factors for cardiometabolic disease among university staff at the University in the Western Cape, Cape Town, South Africa. The secondary objective was to examine the association between the indicators of obesity and CMD risk factors.
Methods:
A total of 73 (men = 20 (27.4%); women = 53 (72.6%)) healthy university staff members, which include academics, administrators, and support staff, with a mean age of 39.2 years partook in the study. Anthropometric and physiological variables were assessed and analysed.
Results:
The categorisation of body mass index showed that 35% and 45.3% of men and women were obese, respectively. The prevalence of central obesity and waist-to-height ratio (WHtR) showed that 25% of men and 60.4% of women were at a high risk of developing a CMD, while 25% and 71.7% of men and women were at high risk for waist-to-hip ratio (WHR), respectively. The prevalence of hypertension revealed that 35% and 20.8%, 5% and 17%, and 15% and 9.4% of men and women had elevated hypertension (stage I) and hypertension (stage II) status, respectively. Furthermore, 25% and 35.8%, and 5% and 11.3% of men and women were pre-diabetic and diabetic, respectively, while for total cholesterol levels, 40% and 34%, and 15% and 15.1% of men and women were at borderline high and high risk, respectively.
Conclusion:
There was a high prevalence of selected cardiometabolic disease risk factors among university staff that requires urgent intervention. Lifestyle modification, weight management, and wellness programmes focusing on health education, regular physical activity participation, and a healthy diet should be prioritized.
1. INTRODUCTION
Cardiometabolic diseases (CMDs) have become the main cause of illness and death worldwide. Variations in the commonness of CMDs and their key risk factors lack consistency throughout settings [1]. The medical disorders in CMDs risk factors are hypertension, insulin resistance, blood lipids, impaired glucose tolerance, and abdominal adiposity [2, 3]. The risk factors for CMD have steadily increased over the years, with many people now being at risk of early infirmity and mortality [4]. The etiology of cardiometabolic risk factors is multi-factorial, especially the interaction between genetics and environment, lifestyle conduct, and socioeconomic class [5].
Cardiometabolic disease is becoming progressively widespread in sub-Saharan African (SSA) nations [6], with the prevalence fluctuating from zero to about fifty percent depending on the inhabitant's location [7]. Data from some SSA countries have revealed a high mortality rate due to CMD, ranging from 17% in Zambia, Mozambique, and Zimbabwe to 36% in Cameroon [8]. The incidence of CMD in SSA could be understood to be motivated by substituting the usual African lifestyle for a Westernised one [9]. Reasons for lifestyle change have been attributed to technological advancement, speedy urbanization, a drop in work-related physical activity, increased ingestion of nutrition that is plentiful in sugar, saturated fats, and salt/processed foods [8, 9].
South Africa is one of the SSA countries that boasts one of the largest economies in the continent of Africa. In South Africa, transmittable and bloodsucking or parasitic diseases, dietary inadequacies, obstetrical and perinatal disorders as well as wound-related situations have dominated the form of ill health and death nationally [7]. However, CMD in SA currently places an increasing burden on many lives and health care systems, with a mortality rate of 34% [8]. Those who are employed by organisations, especially colleges and universities or higher education institutions, are also affected. Furthermore, improvement in their quality of life, health status, and the ability to hold onto healthy conduct are anticipated to have an effect on their throughput, avoid chronic disease occurrences, lower the cost of healthcare, and as a result, enhance the status of the economy of their institution [10].
Comprehensive data on the types and the rate of cardiometabolic risk factors amongst university staff is essential in order to inform the staff at-risk and to design and implement wellness programmes that include routine lifestyle change and physical activity in the prevention and management of CMD factors [11]. To our knowledge, little or no study assessing risk profiles has been conducted among university staff in South Africa. In light of this gap, the current study, therefore, sought to determine the prevalence of CMD risk factors among university staff of the university in the Western Cape. Secondly, the study aimed to examine the association between the indicators of obesity and CMD risk factors.
2. MATERIALS AND METHODS
2.1. Research Design and Sample
A cross-sectional design was used in this study, which was carried out between September 2017 and September 2019. Convenience sampling was used to recruit 73 (men = 20 (27.4%); women = 53 (72.6%)) university staff members, comprising academics, administrators, and support staff, aged 18-63 years. From the original overall sample of 80 staff members assessed, seven participants were excluded due to incomplete data.
2.1.1. Anthropometric Measurements
Participants’ height, body weight, waist (for abdominal obesity), and hip circumferences were assessed according to the procedures of the International Society for the Advancement of Kinanthropometry (ISAK) [12]. The average of three measurements was used for circumferences. From the height and weight, body mass index (BMI) was calculated (kg.m-2) (Eq. 1). The BMI of participants was then used to classify them as underweight (<18.50 kg.m-2), normal weight (18.50-24.99 kg.m-2), overweight (25.0-29.99 kg.m-2), or obese (≥30.0 kg.m-2) [13]. From the circumferences obtained, waist-to-height ratio (WHtR), and waist-to-hip (WHR) were calculated (Eqs. 2 and 3). For WHtR and WHR, a ratio above 0.5 was considered overweight and above 0.95 obese. The cut-off point for abdominal obesity risk was 102 cm for men and 88 cm for women [13].
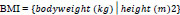
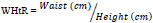
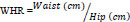
2.2. Physiological Measurements
2.2.1. Blood Pressure
Blood pressure was assessed with the use of a validated electronic device. The measurement was performed by taking three consecutive readings, of which the average of the last two measurements was utilised. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were categorised as normal (<120 and <80 mm Hg), elevated/pre-hypertension (120-129 mmHg and <80 mmHg), hypertension stage one (130-139 mmHg and 80-89 mmHg), hypertension stage two (≥140 mmHg and ≥90 mmHg), and a hypertensive crisis (>180 mmHg and >120 mmHg) [14].
2.2.2. Fasting Blood Glucose
The participants’ fasting blood glucose (FBG) was assessed with validated ACCU-CHEK. Universal health precautions were followed at all times while drawing blood, which was performed by puncturing a finger with a sterile lancet, then wiping away the first drop of blood, and applying the second drop of blood onto a test strip for analysis. Fasting blood glucose was categorised according to the American Association for Clinical Chemistry (AACC) (2020) [15] as below normal (<3.9 mmol/L), normal (3.9-5.5 mmol/L), pre-diabetic (5.6-6.9 mmol/L), and diabetic (≥7.0 mmol/L). Total cholesterol (TC) was measured following the same procedure as for glucose but using the Accutrend® Plus meter for blood analysis. Cholesterol was categorised into normal (<5.2 mmol/L) borderline high or pre-hypercholesterolemic (5.2-6.19 mmol/L) and high (≥6.2 mmol/L) [16]. All physiological variables were measured according to the American College of Sports Medicine guidelines for exercise testing and prescription [13].
2.2.3. Ethics Considerations
Permission to conduct the study was attained from the institutional Ethics Committee (Ethics number: BM17/6/17). Permission was also obtained from the Registrar (Reference number: UWCRP040917SOO) to recruit university staff. Before data collection, the researchers explained the purpose of the study and the procedures to the participants. Only those who voluntarily and verbally agreed to participate were asked to complete and sign a consent form. The Helsinki declaration protocols of 1975, which were reviewed in the year 2013, were also followed.
2.2.4. Data Analysis
Data analysis was performed using the IBM Statistical Package for the Social Sciences (SPSS) version 27.0 (Armonk, NY: IBM Corp). Means, standard deviations, and percentages were used to describe the prevalence of cardiometabolic risk factors among the participants. We then determined the relationship between all dependent variables using Spearman’s rho non-parametric correlation. We followed the interpretation of Dancey and Reidy's [17] categorisations. Before the analysis, a normality test was performed for all variables using the Shapiro-Wilk test. In addition, shaping of the test and visual inspection with a Q-Q plot was performed for all dependent variables. We also performed multiple regression modelling to determine the relative influence of BMI and waist-to-height ratio in predicting abdominal/central obesity, systolic and diastolic blood pressure, fasting blood glucose, and total cholesterol amongst the participants. All statistical investigations were two-tailed and p ≤ 0.05 was considered statistically significant.
3. RESULTS
Table 1 shows the sociodemographic characteristics of the study participants. The majority (60.3%) of the participants were married and had a degree or postgraduate education (38.4%). Most (39.7%) of the participants earned R150 thousand rand or less as their annual income. The most common types of houses lived in were brick houses (82.2%) while the majority (63%) of the participants were the administrative staff. Based on the result of the family history of the participants, 41.1% of the participants reported that they had a family medical history followed by 45.2% who did not. In addition, 13.7% of the study participant reported that they did not know if they had a family medical history.
The prevalence of CMD risk factors amongst the university staff is presented in Table 2. The participants studied had an overall mean age of 39.2 years. Men were significantly taller than women (p<0.001). The overall mean BMI value of the participants fell into the overweight category. Based on BMI, 35% and 45.3% of men and women were obese, respectively. The prevalence of central obesity shows that 25% of men and 60.4% of women were at high-risk levels while 25% and 71.7% of men and women were at high risk for WHRs, respectively. For WHtR, 70% and 73.6% of men and women were above the normal cut-off and were at high risk, respectively. The prevalence of hypertension revealed that 35% and 52.8%, 5% and 17%, and 15% and 9.4% of men and women, respectively had elevated hypertension (stage I) and hypertension (stage II) status. For DBP, the prevalence stood at5% and 3.8%, 30% and 28.3%, and 20% and 22.6% of men and women respectively had elevated hypertension (stage I) and hypertension (stage II) status. Consequently, the prevalence of FBG showed that 25% and 35.8%, and 5% and 11.3% of men and women were pre-diabetic and diabetic, respectively, while for TC, 40% and 34%, and 15% and 15.1% of men and women were at the borderline high and high, respectively.
| Variables | Men | Women | Total | |
|---|---|---|---|---|
| (n %) | (n %) | (n %) | p-value | |
| Marital Status | - | - | - | - |
| Never married | 5 (6.8) | 20 (27.4) | 25 (34.2) | - |
| Married | 15 (20.5) | 29 (39.7) | 44 (60.3) | 0.20 |
| Widow/divorced | 0 (0.0) | 4 (5.5) | 4 (5.5) | - |
| Education Status | - | - | - | - |
| Primary | 3 (4.1) | 10 (13.7) | 13 (17.8) | - |
| High School | 6 (8.2) | 20 (27.4) | 26 (35.6) | 0.85 |
| Diploma/Vocational | 2 (2.7) | 4 (5.5) | 6 (8.2) | - |
| Degree/postgraduate | 9 (12.3) | 19 (26.0) | 28 (38.4) | - |
| Annual Income | - | - | - | - |
| ≤ R150k | 8 (11) | 21 (28.8) | 29 (39.7) | - |
| R151-300k | 6 (8.2) | 18 (24.7) | 24 (32.9) | 0.93 |
| ≥R301k | 6 (8.2) | 14 (19.2) | 20 (27.4) | - |
| House Type | - | - | - | - |
| Bricks house | 16 (21.9) | 44 (60.3) | 60 (82.2) | 0.76 |
| Flat/others | 4 (5.5) | 9 (12.3) | 13 (17.8) | - |
| Nature of Job | - | - | - | - |
| Academics | 5 (6.8) | 13 (17.8) | 18 (24.7) | - |
| Administration | 12 (16.4) | 34 (46.6) | 46 (63) | 0.90 |
| Others (Supt. staff) | 3 (4.1) | 6 (8.2) | 9 (12.3) | - |
| Family History | - | - | - | - |
| Yes | 6 (8.2) | 24 (32.9) | 30 (41.1) | - |
| No | 8 (11.0) | 25 (34.2) | 33 (45.2) | 0.04 |
| Don’t Know | 6 (8.2) | 4 (5.5) | 10 (13.7) | - |
| Variables | Total (n=73) | Men (n=20) | Women(n=53) | p-value |
|---|---|---|---|---|
| Age (y) (M±SD) | 39.2±10.4 | 39.5±13.2 | 39.0±9.36 | 0.89a |
| Age range (yrs.) % | - | |||
| 21-40 | 48 (66) | 13 (65.0) | 35 (66.0) | 0.93b |
| ≥41 | 25 (34) | 7 (35) | 18 (34.0) | |
| Height (m) (M±SD) | 165.8±8.18 | 173.4±7.32 | 163.0±6.51 | < 0.001a* |
| Weight (kg) (M±SD) | 81.2±20.1 | 83.5±16.7 | 80.3±21.3 | 0.51a |
| BMI (kg/m2) (M±SD) | 29.7±7.63 | 27.9±6.39 | 30.4±7.99 | 0.17a |
| Normal weight (%) | - | 7 (35.0) | 15 (28.3) | |
| Overweight (%) | - | 6 (30) | 14 (26.4) | 0.72b |
| Obese (%) | - | 7 (35) | 24 (45.3) | |
| Central Obesity (cm) (M±SD) | 91.7±17.1 | 88.7±19.3 | 92.9±16.2 | 0.39a |
| Low Risk (%) | - | 15 (75.0) | 21 (39.6) | |
| High Risk (%) | - | 5 (25.0) | 32 (60.4) | 0.007b* |
| Hip Circumference (cm) (M±SD) | 109.5±19.1 | 106.7±14.2 | 110.5±20.6 | 0.37a |
| WHR (M±SD) | 0.85±0.23 | 0.83±0.15 | 0.86±0.25 | 0.47a |
| Low Risk (%) | - | 15 (75.0) | 15 (28.3) | |
| High Risk (%) | - | 5 (25.0) | 38 (71.7) | < 0.001b* |
| WHtR (M±SD) | 0.55±0.10 | 0.51±0.11 | 0.57±0.10 | 0.06a |
| Low risk (< 0.5) (%) | - | 6 (30.0) | 14 (26.4) | |
| High risk (> 0.5) (%) | - | 14 (70.0) | 39 (73.6) | 0.75b |
| Body adiposity index (M±SD) | 33.6±10.4 | 28.9±7.19 | 35.5±10.9 | 0.004a* |
| SBP (mm Hg) (M±SD) | 121.3±16.2 | 123.1±19.4 | 120.6±14.9 | 0.61a |
| Normal (%) | - | 9 (45.0) | 28 (52.8) | |
| Elevated (%) | - | 7 (35.0) | 11 (20.8) | |
| Hypertension (stage I) (%) | - | 1 (5.0) | 9 (17.0) | 0.34b |
| Hypertension (stage II) (%) | - | 3 (15.0) | 5 (9.4) | |
| DBP (mm Hg) (M±SD) | 80.3±11.8 | 80.5±10.8 | 80.2±12.3 | 0.93a |
| Normal (%) | - | 9 (45.0) | 24 (45.3) | |
| Elevated (%) | - | 1 (5.0) | 2 (3.8) | |
| Hypertension (stage I) (%) | - | 6 (30.0) | 15 (28.3) | 0.99b |
| Hypertension (stage II) (%) | - | 4 (20.0) | 12 (22.6) | |
| FBG (mmol/L) (M±SD) | 5.81±1.67 | 5.65±1.31 | 5.87±1.80 | 0.56a |
| Normal (%) | - | 14 (70.0) | 28 (52.8) | |
| Pre-diabetic (%) | - | 5 (25.0) | 19 (35.8) | 0.39b |
| Diabetic (%) | - | 1 (5.0) | 6 (11.3) | |
| TC (mmol/L) (M±SD) | 5.51±1.72 | 5.30±0.88 | 5.59±1.95 | 0.38a |
| Normal (%) | - | 9 (45.0) | 27 (50.9) | |
| Borderline high (%) | - | 8 (40.0) | 18 (34.0) | 0.88b |
| High (%) | - | 3 (15.0) | 8 (15.1) |
a=p-value calculated from the independent sample t-test. b=p-value calculated from the Chi-square test.
A bivariate correlation analysis showed that there was a statistically significant relationship between BMI and WC (r = 0.756; p < 0.001), BAI% (r = 0.731; p < 0.001), and SBP (r = 0.258; p < 0.05). There was also a significant correlation between WC and SBP (r = 0.373; p < 0.001), WHR (r = 0.513; p < 0.001), and BAI% (r = 0.689; p < 0.001). Similarly, WHtR was found to be significantly correlated with the WC (r = 0.957; p < 0.001), SBP (r = 0.287; p < 0.05), and BAI% (r = 0.781; p < 0.001) (Table 3).
Table 3.
| Variables | Age | Height | Weight | BMI | WC | HC | WHR | WHtR | BAI | SBP | DBP | FBG | TC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (y) | 1 | - | - | - | - | - | - | - | - | - | - | - | - |
| Height (cm) | 0.041 | 1 | - | - | - | - | - | - | - | - | - | - | - |
| Weight (kg) | 0.024 | 0.069 | 1 | - | - | - | - | - | - | - | - | - | - |
| BMI (kg/m2) | 0.001 | -0.304** | 0.915** | 1 | - | - | - | - | - | - | - | - | - |
| WC (cm) | 0.150 | -0.085 | 0.745** | 0.756** | 1 | - | - | - | - | - | - | - | - |
| HC (cm) | -0.012 | -0.028 | 0.707** | 0.705** | 0.759** | 1 | - | - | - | - | - | - | - |
| WHR | 0.286* | -0.087 | 0.238* | 0.294* | 0.513** | -0.074 | 1 | - | - | - | - | - | - |
| WHtR | 0.114 | -0.328** | 0.681** | 0.794** | 0.957** | 0.736** | 0.478** | 1 | - | - | - | - | - |
| BAI | -0.040 | -0.427** | 0.560** | 0.731** | 0.689** | 0.885** | -0.075 | 0.781** | 1 | - | - | - | - |
| SBP (mm Hg) | 0.210 | 0.162 | 0.343** | 0.258* | 0.373** | 0.319** | 0.123 | 0.287* | 0.198 | 1 | - | - | - |
| DBP (mm Hg) | 0.142 | 0.093 | 0.193 | 0.155 | 0.186 | 0.220 | 0.071 | 0.154 | 0.136 | 0.685** | 1 | - | - |
| FBG (mmol/L) | 0.115 | 0.071 | 0.036 | 0.021 | 0.133 | 0.105 | 0.004 | 0.137 | 0.093 | 0.188 | 0.149 | 1 | - |
| TC (mmol/L) | 0.223 | -0.008 | 0.160 | 0.184 | 0.173 | 0.041 | 0.154 | 0.085 | 0.079 | 0.094 | -0.059 | -0.067 | 1 |
** Correlation is significant at the 0.01 level (2-tailed).
* Correlation is significant at the 0.05 level (2-tailed) [1].
A linear regression analysis was calculated to predict each of the cardiometabolic diseases (abdominal obesity, systolic blood pressure, diastolic blood pressure, fasting blood glucose, and total cholesterol) based on the BMI and WHtR (Table 4). For WC, a significant regression equation was found (F(1, 71) = 78.989, p < 0.001), with an R2 of 0.527. Participants’ predicted WC is equal 43.284 + 1.631 (BMI) centimetre when BMI is assessed in kg/m2. Participant’s WC increased by1.631 for each kilogram per meter square of BMI. Similarly for TC, a significant regression equation was found (F(1, 71) = 4.302, p = 0.042), with an R2 of 0.057. Participants’ predicted total cholesterol is equal 3.905 + 0.054 (BMI) mmol/L when BMI is assessed in kg/m2. Participant’s TC increased 0.054 for each kg/m2 of BMI. Based on the WHtR, a significant regression equation was found (F(1, 71) = 1099.199, p < 0.001), with an R2 of 0.939. Participants’ predicted WC is equal 7.172 + 152.382 (WHtR) centimetre, when WHtR is assessed in centimetre. Participant’s WC increased by 152.382 for each centimetre of WHtR.
| Dependent variable | Variable in the model |
Unstandardized
Coefficient |
p-value | R2 | Adjusted R2 | F-value |
|---|---|---|---|---|---|---|
| WC | (constant) | 43.284 | 0.000 | 0.527 | 0.520 | 78.989 |
| BMI | 1.631 | 0.000 | - | - | - | |
| SBP | (constant) | 111.616 | 0.000 | 0.024 | 0.010 | 1.711 |
| BMI | 0.326 | 0.195 | - | - | - | |
| DBP | (constant) | 75.861 | 0.000 | 0.009 | -0.005 | 0.667 |
| BMI | 0.150 | 0.417 | - | - | - | |
| FBG | (constant) | 5.316 | 0.000 | 0.006 | -0.008 | 0.410 |
| BMI | 0.017 | 0.524 | - | - | - | |
| TC | (constant) | 3.905 | 0.000 | 0.057 | 0.044 | 4.302 |
| BMI | 0.054 | 0.042 | - | - | - | |
| WC | (constant) | 7.172 | 0.007 | 0.939 | 0.938 | 1099.199 |
| WHtR | 152.382 | 0.000 | - | - | - | |
| SBP | (constant) | 105.872 | 0.000 | 0.035 | 0.021 | 2.575 |
| WHtR | 27.793 | 0.113 | - | - | - | |
| DBP | (constant) | 71.941 | 0.000 | 0.019 | 0.005 | 1.395 |
| WHtR | 15.084 | 0.242 | - | - | - | |
| FBG | (constant) | 4.788 | 0.000 | 0.014 | 0.000 | 1.033 |
| WHtR | 1.843 | 0.313 | - | - | - | |
| TC | (constant) | 4.123 | 0.000 | 0.025 | 0.011 | 1.824 |
| WHtR | 2.507 | 0.181 | - | - | - |
4. DISCUSSION
This study examined the prevalence of CMD risk factors among staff members at the University of the Western Cape. Roth et al. [18] highlighted three major parameters that contribute to or propel CVDs. These could either be based on the behaviours, cardiac and metabolic events, environs, and social determinants [18]. Universities and institutes of higher learning working environment and staff behaviours are of no exemption. Consequently, it has been reported that cardiometabolic disease is an important cause of work-related mortality and has become difficult to track and manage, especially in the workplace [3]. Further studies have reported a continuous rising of an increase in the prevalence of cardiometabolic diseases among staff members of universities [19, 20].
Our findings showed that there was a high prevalence of cardiometabolic disease risk factors among the study participants. Our study revealed a high prevalence, by risk level, for abdominal obesity, waist-to-hip ratio, and waist-to-height ratio among the studied participants, especially women. Women seem to have been severely implicated when it comes to gender differences with a high prevalence of cardiometabolic risk globally being one of the major factors behind disability-adjusted life-years (DALY) lost [21]. Although, de Jong et al. [22] reported that cardiometabolic risk features in females might not be the same when compared to their male counterparts due to the higher body mass index regularly noticed in females. Separate from body mass index, central or abdominal obesity has been reported as an influential parameter causing depreciation to cardiovascular functioning in individuals [23-31]. The present study also indicates a high prevalence of hypertension for both systolic and diastolic [14, 23] blood pressure. One Ethiopian institutionally based cross-sectional study including university staff found a high prevalence of hypertension among the study participants [32]. Chinyere et al. [33] and Rampal et al. [34] also found a high prevalence of hypertension among tertiary institution employees. The increased risk of hypertension has been understood to start in the elevated stage [2, 35] and continues to increase due to the increase in body weight with age [36].
Our study findings also revealed a high prevalence of fasting blood glucose and cholesterol levels. High fasting blood glucose or diabetes mellitus is a metabolic irregularity and a risk factor for impaired vision, cerebrovascular disease, the inability of the kidney to remove waste and balance fluids, and amputations [37, 38]. On the other hand, high cholesterol levels have been linked to atherosclerosis, causing major constriction of the arteries and reduced blood distribution to the brain (and other tissues), resulting in stroke or cerebrovascular attack [39]. Lifestyle changes involving regular physical activity and healthy diet consumption have been established to be effective in its prevention and management [40-44].
The findings from the bivariate analysis showed that there was a significant relationship between BMI and abdominal obesity and between BMI and systolic blood pressure. Furthermore, the waist-to-height ratio was found to be significantly correlated with abdominal obesity and systolic blood pressure. One Chinese study also found a significant correlation between BMI and related cardiometabolic disease risk factors [45]. Body mass index equal to or higher than 25kg/m2 for overweight and 30kg/m2 for obesity has been implicated in mostly predisposed an individual to some illnesses compared to the individual who fell within the normal BMI range [46]. Possessing higher BMI continues to be a major predictor of all five main CMD risk factors, especially hypertension [46].
The findings from the regression analysis revealed a significant increase in abdominal obesity. Participant’s abdominal obesity increased by 1.631 for each kilogram per meter square of body mass index. Similarly, the participant’s total cholesterol increased by 0.054 for each kg/m2 of the body mass index. This denotes that the higher the body mass index, the higher the abdominal obesity and total cholesterol. For systolic blood pressure, diastolic blood pressure, and fasting blood glucose, the prediction model was not significant while the regression equation was not reported. Similarly, participant’s abdominal obesity increased by 152.4 for each centimetre of waist-to-height ratio. A high body mass index remained a major predictor of all five cardiometabolic disease risk factors [2, 46] especially abdominal obesity and systolic blood pressure.
This study represents the first of its kind, which seeks to examine the prevalence of CMD risks factor among university staff in the Western Cape. Although few studies have been conducted in the area of cardiovascular disease risk factors among staff at a tertiary institution in South Africa, but not specifically on cardiometabolic disease risk factors. The results of this study offered credibility to the need for a regular health screening, coupled with obesity management and disease prevention programmes for university employees. This may include diverse wellness programmes, including creating awareness about healthy diets and regular physical activity to improve overall well-being. It is a necessity that universities administrators and managers create good health policies that seek to improve the overall health of the university staff members. The findings of this current study should be cautiously elucidated and should not be broadly viewed due to some limitations; (1) the small number of participants but provides data that could be valuable in tackling the rising trend of cardiometabolic disease risk factors among university employees. (2) A low level of assurance of participants complying with the fasting period could influence blood sugar level prevalence. (3) We did not separate data for academics, administrators, and support staff, considering the relatively small number of participants in this study.
CONCLUSION
The findings of this study revealed a high prevalence of cardiometabolic disease risk factors among employees of the University of the Western Cape. The study also showed that the body mass index and waist-to-height ratio were predictors of cardiometabolic disease risk factors among this population. Further studies involving more participants are recommended to investigate these factors further.
LIST OF ABBREVIATIONS
| CMDs | = Cardiometabolic diseases |
| ISAK | = International Society for the Advancement of Kinanthropometry |
| SSA | = Sub-Sahara Africa |
| BMI | = Body Mass Index |
| WHtR | = Waist – to – Height - Ratio |
| WHR | = Waist-Hip Ratio |
| AACC | = American Association for Clinical Chemistry |
| TC | = Total Cholesterol |
| SBP | = Systolic Blood Pressure |
| DBP | = Diastolic Blood Pressure |
| WC | = Waist Circumference |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The research was approved by the University of Western Cape Research Ethics Committee (Ethics number: BM17/6/17) and the Registrar (Reference number: UWCRP040917SOO) to recruit university staff.
HUMAN AND ANIMAL RIGHTS
No animals were used for studies that are base on this research. All the humans used were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
All the participants who participated were informed verbally about the study and those who gave written informed consent were enrolled.
STANDARDS OF REPORTING
STROBE guidelines and methodology were followed.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
The funding was provided by the National Research Foundation (NRF) of South Africa for providing S&F - DST / NRF Freestanding Postdoctoral Fellowships Grant (Grant reference/Number SFP180413320381/116715) support to Dr. SO Onagbiye.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors acknowledge the opinions, findings, conclusions, and recommendations expressed in this paper are those of the authors, and the funders accept no liability whatsoever in this regard.


