All published articles of this journal are available on ScienceDirect.
Larval Morphology and Molecular Identification of Ophthalmomyiasis Flies and its Incidence Rate in Referred Patients to an Ophthalmology Clinic, Shiraz, Iran
Abstract
Background:
Myiasis is the infestation of flies' larvae in living or dead tissues of the human body and animals. Ophthalmomyiasis is divided into internal and external types and thelarvae penetrate eyes in the internal type. This study aimed to examine larval morphology and molecular identification of flies causing ophthalmomyiasis and determine its incidence rate in referred patients to an ophthalmology clinic in Shiraz during 2019.
Materials and Methods:
During one year, all larvae were isolated from patients’ eyes. These larvae were identified using the morphological method according to the 1965 diagnostic key of Zumpt. Molecular confirmation was performed using a pair of specific primers for the Cytochrome Oxidase I (COI) gene in the next step. The expected amplicons were sequenced, and their results were aligned and analyzed using the nucleotide BLAST.
Results:
Overall, 224 fly larvae were isolated from 36 patients. According to the morphological analysis, all larvae were Oestrus ovis. Molecular analysis confirmed morphological results. Patients presenting acute conjunctivitis symptoms had a mean age of 34 ± 2 years, and there were four women (11%) and 32 men (89%). The highest incidence were recorded in the autumn season (55.8%). Morphological results of flies were confirmed by amplifying the expected size of the CO1 gene through conventional PCR.
Conclusion:
The cases of ophthalmomyiasis are higher than those mentioned in the published articles and this might be due to the absence of a regular monitoring program to care for the ophthalmomyiasis cases in the public health system. Therefore, due to the importance and potential incidence of this disease in Fars province, establishing a disease care program is necessary for the health surveillance system.
1. INTRODUCTION
An infestation of human or animal tissues with larvae of blowflies is called Myiasis. The word "myiasis" is derived from the Greek root myia which means to fly. Cutaneousmyiasis is the most common clinical form. It is also possible to get through body's natural cavities such as the mouth, ears, eyes, nose, sinuses, pharynx, and genitourinary tract. Myiasis is classified into entomological and clinical types. Entomological myiasis is classified according to the parasitic traits of larvae. These characterizations include obligatory, facultative, and accidental myiasis. Clinical myiasis is classified based on the anatomical region in which the infestation occurs [1]. Examples of clinical types include ophthalmomyiasis [2], wound myiasis [3], pharyngeal myiasis [4] ear myiasis [5], urogenital myiasis [6] auricular myiasis [7], oral mucosa myiasis [8], and orbit myiasis [9].
Furthermore, clinical sorting of myiasis may be reported as primary or secondary. In primary myiasis, fly larvae invade living tissues, and they are observed in human and animal eyes, occasionally and abundantly, respectively. Secondary myiasis is developed when fly larvae invade and feed on the dead tissue (necrophagous), which is usually seen in suffering patients from chronic cavity ulcers [10].
Commonly, ophthalmomyiasis is observed in less than 5% of all myiasis cases in the world [11]. The most frequent ophthalmomyiasis cause is Oestrus ovis from the family of Oestridae. Domestic animals (sheep and goats) are the natural hosts of their larvae, but this botfly also attacks humans occasionally. The prevalence of myiasis depends on climatic and ecological factors and the population of susceptible flies and animals. Early spring and summer to autumn, usually associated with tropical and subtropical conditions, poor hygiene, and housing conditions, provide a suitable situation for myiasis occurrence. Sometimes, a larva invades the eyeball (anterior vitreous chamber, retina), thereby causing blindness [12-14]. Eye diseases have been reported in different parts of the world, including some European countries, North America [15], Africa [12], Australia [16] and Asia (Iran [14], Pakistan [17], Afghanistan [18], Kuwait [19], Jordan [2], Saudi Arabia [20], and Iraq [14]).
Fars province, Iran, with about 12 million livestock units, is known as the second livestock pole in Iran, and multiple cases of ocular myiasis, especially among the ranchers, are reported. This study was conducted to examine larval morphology and molecular identification of ophthalmomyiasis flies and their incidence rate in referred patients to an ophthalmology clinic in Shiraz, Iran, during 2019.
2. MATERIALS AND METHODS
2.1. Study Area
Fars province lies (29° 37′ 0″ N, 52° 32′ 0″ E) in the southwestern part of Iran. The climate of this province is divided into: mountainous, temperate, and warm climates. The center of this province is Shiraz (29° 37′ 0″ N, 52° 32′ 0″ E), which is the most populous city of this province and the fifth most populated city of Iran. The various tribes: Fars, Baseri, and Qashqai, live in this province, and their important economic resources are agriculture and animal husbandry (Fig. 1).
2.2. Sample Collection
Samples were collected from referral patients to the ophthalmology clinic of “K” hospital during 2019. All patients with symptoms of pain, burning, and inflammation of the eye, redness, and feeling of a foreign body in the eye are referred to this ophthalmology clinic in Shiraz, Iran. Then, their checklists were completed, and related data of age, sex, place of residence, occupation, number of larvae, and type of referral were documented. Fly larvae were removed from the eye using tetracycline drops and forceps, placed in 70% ethanol vials, and then transferred to the Department of Medical Entomology Laboratory, School of Health, Shiraz University of Medical Sciences (SUMS) for morphological and molecular identification.

2.3. Morphological Identification
According to the diagnostic key for the third stage larva, the blowfly larvae were studied for morphological specifications using the stereomicroscope (Zumpt, 1965). The larvae were mounted on a glass slide using the Puri's medium and examined under the Stereomicroscope (Olympus 50× zoom, Japan). The larvae's most important diagnostic features are the cephalo-pharyngeal skeleton, lateral spines, and posterior spiracles.
2.4. Molecular Identification
According to the manufacturer's instructions, DNA from whole larvae was extracted using the DNA extraction kit (GenAll DNA isolation kit, South Korea). The final volume of the extracted samples was 100 μl, which was stored at -20 C. The primers were designed based on the cytochrome oxidase I (COI) gene of the mitochondria with AF257118.1 Sequence ID in GenBank. For species confirmation OseF: 5′- ACTATTA GTAAGAAGAATAG-3′and OseR: 5′ AAGTTGCAGGAGA GTAGTTG -3′ primers were used. PCR reactions with specifically designed primers were done to perform molecular characterization of the collected samples. The final volume of PCR mixtures was 20μl and contained 0.2μl Taq DNA polymerase, 8μl of 2.5X Mastermix, 9.8μl DNase/RNase-free Double Distilled Water (DDW), 1μl of 10 pmol/μl of each primer, and 1μl of DNA template. All PCR ingredients were purchased from Sinaclone (Iran). A negative control (no template control: NTC) was considered in each run. PCR program was as follow: 5 min at 94°C as initial denaturation and 35 cycles of the 30s at 95°C, 30s at 56°C, and 30s at 72°C, with a final extension for 5min at 72°C. All PCR products were subjected to agarose gel electrophoresis in a 0.5× Tris-Acetate EDTA buffer. The amplified fragments were visualized after staining using the DNA safe stain (Sinaclon, Iran) with UV light. The agarose gel's expected size bands were recovered using the GF1 Gel DNA recovery purification Kit (Vivantis, Malaysian) following the manufacturer's instructions. Then, sequencing in both directions was done based on the forward and reverse GSP primers with Iran's Pishgam Company. The sequence reads were assembled using the GeneStudio Pro Software v.2.2.0.0), and the consensus file was aligned and analyzed using the nucleotide BLAST web-based software (https://blast.ncbi.nlm.nih.gov/Blast.cgi) and the genus and species of the samples were determined according to their similarity with the deposited sequences in GenBank.
3. RESULTS
In total, 36 patients of referred patients to the ophthalmology clinic of “K” hospital of Shiraz had ophthalmomyiasis, 32 (88.88%) cases were male, and 4 (11.12%) were female. Most cases were in the age range of 26-40 years, and the frequency of this group was 23 patients (63.9%). The mean age of patients was 34 ± 2 (mean ± SD) (Table 1).
| Age | 10-25 | 26-40 | 41-55 | 56-70 | Total |
|---|---|---|---|---|---|
| Female | 1 | 2 | 1 | 0 | 4(11.12%) |
| Male | 4 | 21 | 4 | 3 | 32(88.88%) |
| Total | 5(13.9%) | 23(63.9%) | 5(13.9%) | 3(8.3%) | 36(100) |
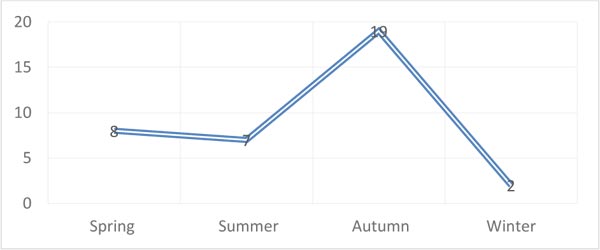
There was no case in the age group of less than ten years. The present study showed that the highest occurrence with the incidence of disease was associated with autumn (19 cases) and the lowest in the winter (2 cases) (Fig. 2). The number of isolated larvae from patients was from 1 to 19 larvae. All patients had unilateral ocular disorder. They stated that while doing work or walking in the street, after being hit in the eyes by the flies, they immediately felt burning, pain, redness, and tears. An eye examination with a slit lamp showed mild edema of eyelids and the presence of occasional pseudo-membranes. The larvae' length was about 1-2 mm, and they had a pale appearance with a blackhead (cephalo-pharyngeal skeleton). They were sensitive to light and, when exposed to slit light, were hidden under the eyelids or conjunctivitis folds.
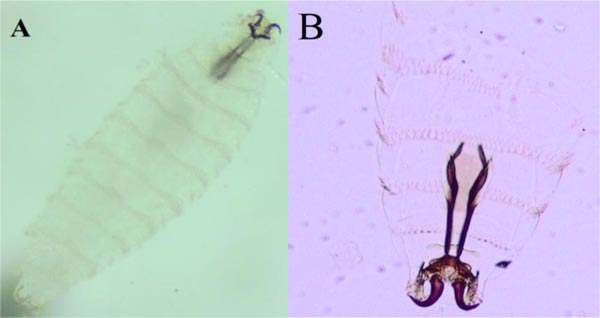
A total of 224 larvae were collected in this study. All of them were identified as Oestrus ovis based on their spindle shape and the presence of a pair of sharply curved mouth hooks, the presence of a series of spines on each segment's abdominal surface, large outwardly-pointed oral hooks on spherical bases and no trunks (Fig. 3).
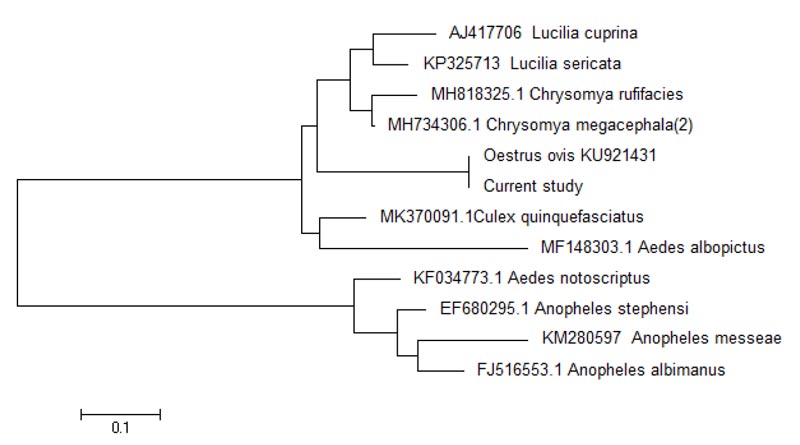
For molecular identification, PCR assays were performed with OeF and OeR primers according to section 2.4. above. The expected size band was amplified and then sequenced by Pishgam, Iran Company, and results were registered in GenBank No. MW316742. The alignment and nucleotide BLAST analysis revealed that all aligned sequences had a 100% match to deposited COI gene subjects from Oestrus ovis in GenBank by other researchers. A phylogenetic tree was constructed according to the COI gene sequence of Lucilia sericata, Lucilia cuprina, Chrysomya rufifacies, Anopheles stephensi, Culex quinquefasciatus, Aedes albopictus, Aedes notoscriptus, Chrysomya megacephala, Anopheles messeae, Oestrus ovis and our acquired sequence using the maximum likelihood method. Phylogenetic analysis showed that the highest similar physiological function and evolutionary relatedness was related to the COI subunit 1 of Oestrus ovis (Fig. 4).
4. DISCUSSION
In this study, 224 larvae were isolated from 36 patients. According to the morphological analysis, all larvae belonged to the genus and species of Oestrus ovis, and molecular analysis confirmed the morphological results. Three dipteran families are considered to be the leading cause of myiasis. Oestrus ovis (sheep nasal botfly) from the class Insecta, order Diptera and family Oestridae, is one of the most common agents of human and animal myiases reported in the world [21-25]. Other species that cause human myiasis are Dermatobia hominis (human botfly) and Cordylobia anthropophaga (tumbu fly) [26-29].
In this study, 36 cases of ophthalmomyiasis were referred to “K” hospital during 2019. This study showed that 88% and 11% of the patients were male and female, respectively. Similar studies have reported that the percentage of ocular myiasis is higher in men [27, 30]. The reason can be related to men's jobs, for example, agricultural and livestock jobs. Most clients were related to suburban regions. In 2004, Massoudi et al. showed that men are more likely than women to have the ophthalmomyiasis disease in Fars (Iran) [31]. The present study showed that most cases were in autumn. This finding is also shown by Taouri in 2020 [32], therefore it can be conceived due to favorable weather conditions for the abundance of flies in this season and coincided with the harvest of agricultural products in Fars. The disease has occurred in the fall, and it is suggested that local health officials prepare plans to control flies in this season.
In the present study, most of the patients were 26-40 years old (mean 35 years), followed by 41-55 and 10-25 year-old age groups with frequencies of 63.9%, 13.9%, and 13.9%, respectively. The lowest frequency was related to the 56-70-year-old (8.3%), and no cases were seen in the less than 10-year-old age group. These results are similar to the study by Ayatollahi et al. that was conducted in Yazd in 2014, with the highest frequency in patients being 34 years old [33]. The average number of removed larvae from each patient was ten larvae in this study. In the study by Ayatollahi et al., the average number of isolated larvae from each patient was 5 [33]. This study reports ophthalmomyiasis fly such as Oestrus ovis in Iran. Furthermore, other studies were conducted by some researchers around the world who reported that myiasis flies in tropical regions, mainly in Africa, and Asia are distributed in the Southern Hemisphere [34].
Shiraz city has a suitable climate for myiasis flies. Several studies have reported myiasis flies in coastal areas [35]. Besides, the prevalence of myiasis is dependent on climatic, ecological factors, the population of flies, and the presence of sensitive animals [36]. Flies are seen in the early of spring, summer, and fall in Shiraz city. Oestrus ovis in Shiraz city is usually associated with tropical and subtropical areas, poor hygiene, and housing conditions. Eye diseases (Ophthalmomyiasis) have been reported in different parts of the world, including some European countries, north of America, Africa, Australia, and Asia (Iran, Pakistan, Afghanistan, Kuwait, Jordan, Saudi Arabia) [37-41].
Furthermore, external ophthalmomyiasis could be caused by Oestrus ovis in humans. The Oestrus ovis fly causes myiasis in many domestic animals and causes many economic losses [27, 42]. This fly is also called the nasal botfly of sheep and, in the traditional language of Fars, is called Cyspoo. Most cases of human myiasis occur in the eyes, which could also cause conjunctivitis in the eyes. Besides, in some cases, it may lead to blindness. Concerning favorable weather conditions, Fars province is considered one of the livestock areas in the southern part of Iran. Several cases of myiasis from this province are reported each year. Oestrus ovis is capable of causing the external ophthalmomyiasis [43]. Moreover, Fars province's climate is suitable for the other vector-borne diseases such as malaria [44, 45] and leishmaniasis [46, 47], and related cases have been reported from this province in previously performed studies.
Concerning the importance of ophthalmomyiasis and related disorders for the ocular system, rapid and precise identification of larvae is essential to decide the correct treatment and alarm the health care system. One of the limitations in this study was the collection of samples because, in the clinic system, they had no obligation to report such cases; therefore, most samples are discarded without saving or recording. Finally, it is recommended that ophthalmologists remind their students of necessary training on the public health significance of this disease.
CONCLUSION
This study shows that due to climate change, neglected diseases have become common again, and ophthalmologists and health centers should pay attention to this issue and endeavor to upgrade medical students' training. Also, health care systems should prioritize this disease to control planning.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was performed following the Iranian National Committee for Ethics guidelines in Biomedical Research and was approved by the Ethics Committee of SUMS (IR.SUMS.REC.1398.341).
HUMAN AND ANIMAL RIGHTS
No Animals were used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Written consent was acquired from every patient included in this research.
AVAILABILITY OF DATA AND MATERIALS
The information used and analyzed during the present study is available from the corresponding author upon reasonable request.
FUNDING
Shiraz University of Medical Sciences funded this project, with grant number 97-01-04-17592, to Dr. Hamzeh Alipour for Mr. Ali Keshavarz as part of his M.Sc. thesis in Medical Entomology.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
We would like to thank the staff of “K” Hospital and Department of Medical Entomology, School of Health, Shiraz University of Medical Sciences, Shiraz, Iran.


