All published articles of this journal are available on ScienceDirect.
Combating the COVID-19 Pandemic Using 3D Printed PPE: Challenges and Recommendations
Abstract
Background:
The World Health Organization declared COVID-19 a pandemic in March of 2020. As traditional respiratory personal protective equipment (PPE) was in severe shortage, communities turned to 3D printing to provide printed PPE alternatives; however, certain hurdles need to be addressed to ensure the safety of users.
Objective:
One main consideration when dealing with 3D printed parts is the presence of pores. Several studies have found the diameter of these pores to range widely from as little as 10µm to over 150 µm, making them larger than the droplets and nuclei through which the virus is transmitted.
Methods:
Researchers found that altering print settings, such as increasing the extrusion multiplier, may decrease the size and number of these perforating pores. Other challenges include the variable reproducibility of printed PPE, which may be remedied through printer calibration. Storage and sterilization are also a challenge as most 3D printed plastics do not tolerate disinfection methods, such as autoclaves. The use of chemical disinfectants is recommended instead. The rigidity of printed plastics may compromise the fit of masks for varying users. Using 3D scanning may provide personalized masks that seal appropriately.
Results:
One final issue is the prolonged interaction with 3D printers of inexperienced users, predisposing them to the respiratory tract and skin irritation; thus, adequate ventilation and protection are mandatory.
Conclusion:
Documenting the benefits and drawbacks of this form of PPE production carries great significance in light of the ongoing COVID-19 pandemic, as well as any future public health emergencies.
1. INTRODUCTION
On December 31st, 2019, clusters of pneumonia cases were reported in Wuhan, China; three months later, the novel coronavirus, COVID-19, was declared a pandemic by the World Health Organization (WHO) [1]. Due to the crushing demands, supply of traditional respiratory personal protective equipment (PPE) quickly became limited. One solution to combat this mismatch emerged in the form of 3D printed PPE. 3D printing has proved to be a suitable interim replacement in times of need due to easy access to 3D printers and design software, the abundance of material choices, and the reusability of printed parts. 3D printing has played a crucial role in fulfilling the PPE demands during shortages experienced at the beginning of the COVID-19 pandemic. According to the United States Food and Drug Administration (FDA), additive manufacturing has contributed to producing approximately 53 million PPE, PPE accessories, and medical devices [2]. Despite this, there remain challenges that need to be addressed, as cited previously in statements released by the FDA [3]: “While it is possible to use 3D printing to make certain PPE, there are technical challenges that have to be overcome.”
2. SYNOPSIS OF THE WIDESPREAD IMPACT OF 3D PRINTED PPE
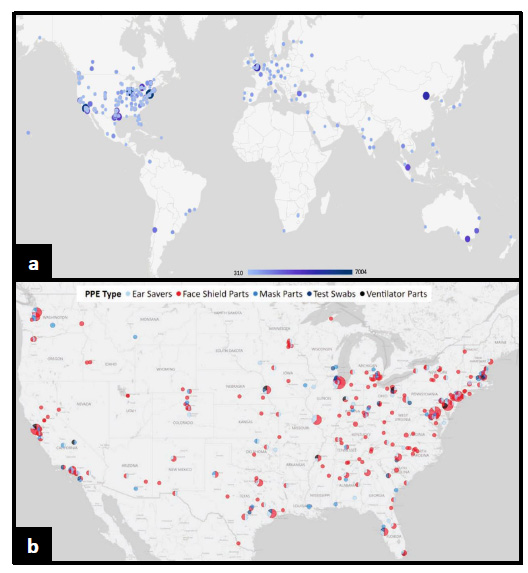
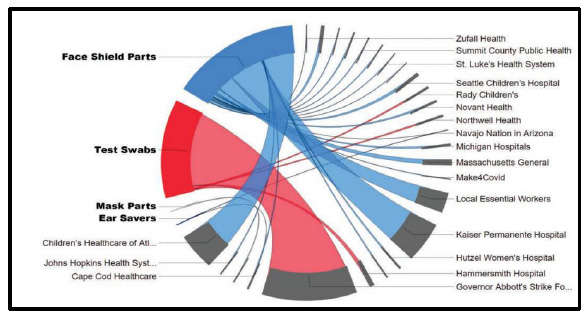
In response to the sudden supply chain shortages of traditional PPE, non-traditional producers (NTP) were quick to design and produce 3D printed PPE in large quantities. While exact production estimates may vary, one study found that over the course of 5 months in the United States alone, 53 million 3D printed PPE and medical devices were produced utilizing more than 33,000 3D printers. The majority of printed PPE came in the form of face shields, which amounted to 38,295,580 units produced in total with a maximum daily production capacity of 502,593 units. Test swabs were the second-largest produced 3D printed medical devices with 12,376,896 total units produced and a maximum daily capacity of 948,204 units. Ear savers, masks and mask parts, and ventilator parts were the least produced items, with an estimated total production of 2,560,954 units, 241,869 units, and 116,455 units, respectively [2]. The distribution of said 3D printed PPE was in concordance with the intensity of COVID-19 infection rates in different geographical areas (Fig. 1). Examples of different recipients of printed PPE from NTPs in the United States include Massachusetts General Hospital, the Johns Hopkins Health System Corporation, Northwell Health, Seattle Children’s Hospital, Novant Health, and Michigan Hospitals, among others (Fig. 2). During this time, the “NIH 3D Print Exchange” program was established wherein the National Institute of Health (NIH), United States Department of Veterans Affairs (VA), America Makes, and FDA, all participated in testing and validating various 3D designs of PPE uploaded by the community. The testing criteria used were based on an amalgamation of standards and regulations from the FDA, Center for Disease Control, and National Institute for Occupational Safety and Health. In total, 645 designs were uploaded, with 33 and 28 designs approved for clinical use and community use, respectively [4-7]. 25% of NTPs used approved 3D printed PPE designs from the NIH 3D Print Exchange program making up the majority of alternative PPE produced for essential workers and healthcare personnel [2]. As such, tested and verified printed PPE had a worldwide reach with online visitors browsing the NIH 3D Print Exchange programs website from over 1000 cities across the globe (Fig. 1) [8]. Whilst initially being met with reluctance, 3D printed PPE had now started to become widely accepted in the community for a multitude of reasons. This, in part, was due to the fact that 3D printers are relatively easy to access and can produce complex geometrical shapes with a high degree of precision, and also due to their ability to use a host of different plastic filaments, which can give the PPE varying physical properties. 3D printing can also work to enhance traditional PPE, such as in the case of mask fitters and mask tensioners. 3D printed PPE is also reusable and can be disinfected more easily, unlike most traditional PPE. This is especially noteworthy as a study conducted in 2020 surveying over 23,000 nurses in the United States reported that 87% of nurses complained of having to reuse a single-use disposable mask or N95 respirator during the first couple of months of the pandemic [9].
3. CHALLENGES AND ASSOCIATED RECOMMENDATIONS RELATED TO 3D PRINTED PPE
3.1. Porosity and Mask Integrity
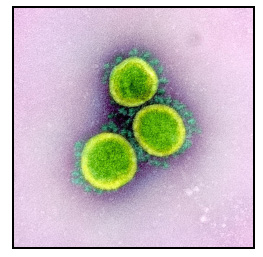
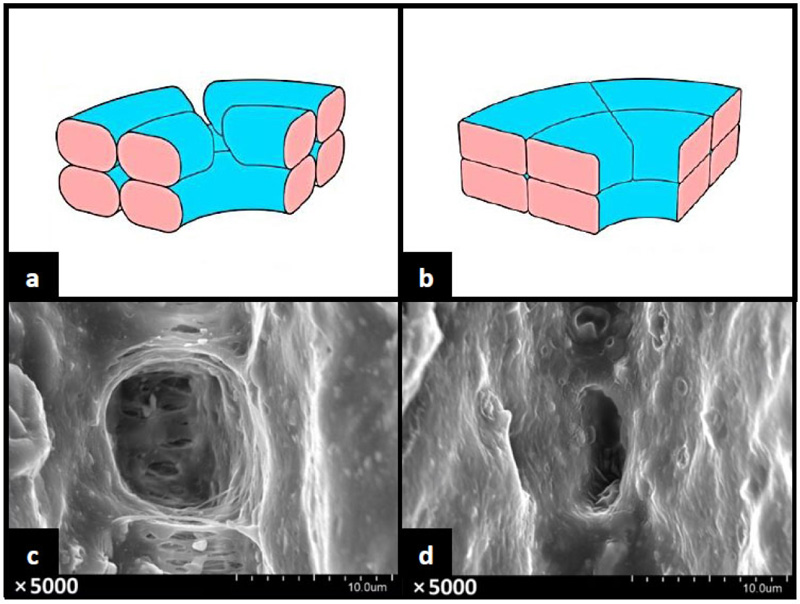
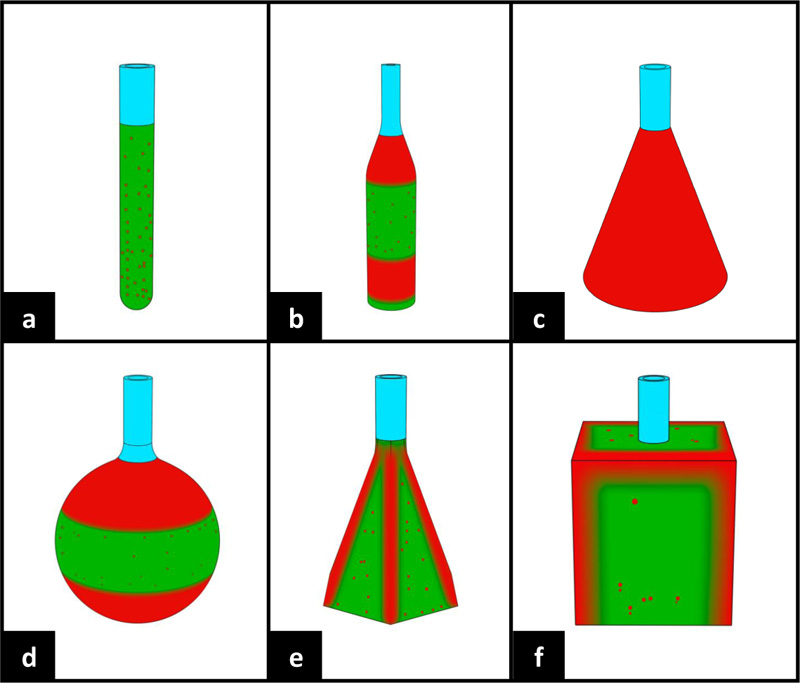
Fused Deposition Modelling (FDM) printers are some of the most widely used types of 3D printers in both hobbyist and commercial settings alike [10]. However, the main shortcoming of FDM printing is the relatively low production quality coupled with the porous nature of the finished print. This may be problematic when involving respiratory PPE production as pores may affect the PPE performance as air and fluid barriers. The COVID-19 virus, known as SARS-CoV-2, was found to range in diameter from 0.065µm-0.125µm (Fig. 3) [11, 12]. Although, worth noting is that the virus particles, as reported by the WHO, are transmitted primarily through respiratory droplets ranging in size between 5µm-10µm. The WHO also reported the potential of transmission through airborne nuclei <5µm in specific settings, such as aerosol-generating procedures or in small, indoor, and poorly ventilated areas [13]. One study found that objects printed with FDM printers contain chaotically arranged pores averaging in size approximately between 10µm-20µm, making them larger than the virus-containing droplets and nuclei [14]. The researchers of the said study were then able to alter the porosity by readjusting printing parameters, such as increasing the extrusion multiplier, allowing for internal filling of the printed walls and distribution of seams for each layer in random positions. Increasing the extrusion multiplier showed to have the largest effect on the porosity of the printed parts. After analysing the modified parts via a scanning electron microscope (SEM), the researchers revealed a reduction in pore size and the absence of any perforating pores (Fig. 4). This provides promising data in the search to eradicate pores in printed parts. However, the researchers indicated varying results depending on the different geometry of the 3D printed components (Fig. 5). Simple geometrical shapes were tested and displayed selected areas of increased porosity. Despite the availability of such data, reducing porosity in complex shapes, such as face masks, may yet be challenging. Another study concerning a different method of 3D printing termed Selective Laser Sintering (SLS) also found the presence of pores. These pores ranged in size from 15µm-150µm [15]. This study did not conduct a follow-up test after adjusting the print settings. The pores in the 3D printed material in both studies were significantly larger than those found in a variety of traditional PPE, such as certified N95 masks, which are rated at 0.3µm [16]. However, this porosity may not be of great concern in regards to PPE used in environments where airborneprecautions do not apply such as in community settings and non-specialized clinical settings (Fig. 6).
3.2. Variable Reproducibility
Despite the fact that the digital and downloadable standard tessellation (STL) files of 3D printed PPE do not differ from one computer to another in terms of printing, these STL files will, more often than not, result in printed PPE with slightly varying dimensions across different 3D printers. This is due to dissimilar printer calibration levels as well as small differences in the generated g-code from different slicing software and print settings. The end result may be an aesthetically similar, but functionally different set of PPE [17]. Furthermore, varying materials may also increase functional differences in the printed products. This lack of quality control was stated as a significant reason behind the inability to ensure the quality, safety, and efficacy of 3D printed PPE by the NIH, VA, and FDA [18]. As such, it is recommended to ensure adequate printer calibration and adhere to the print settings suggested by the original designer of any proficient 3D printed PPE model.
3.3. Heat Sensitivity and Sterilization
FDM printing involves a wide selection of thermoplastic filaments, with polylactic acid (PLA) being a popular choice among the users. This is due to its cost-efficiency, widespread availability, and high print success rates when using basic 3D printers with and without heated build plates. However, printed PLA components are more sensitive to heat compared to other printed plastics. While PLA does have a melting temperature between 130˚C – 180˚C, the glass transition temperature for amorphous PLA lies only between 55˚C – 60˚C, wherein the plastic becomes soft and may warp [19]. This glass transition temperature increases slightly in semicrystalline PLA. This property of PLA is especially problematic for common hospital sterilization methods involving high temperatures, such as using dry heat and autoclaves, which regularly operate at 121˚C or 132˚C [20]. The WHO, therefore, recommends sterilization of plastic respiratory equipment that may not tolerate high temperatures, such as PLA printed PPE, by using chemical disinfectants and soaking in a 0.1% sodium hypochlorite solution for most cases. For cases of equipment exposed to over 10ml of blood or body fluid, a higher concentration of 0.5% is recommended [21-23]. Such chemical disinfectants require cautious use to avoid wear on the printed PPE.
3.4. Ergonomics and Seal
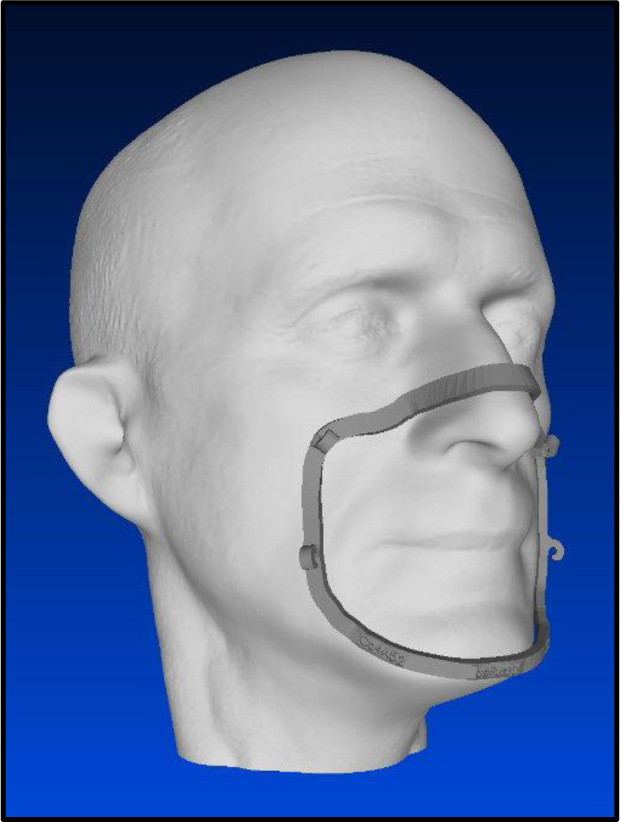
While alternative plastics may overcome the aforementioned temperature barriers, they still share other drawbacks with PLA printed PPE, such as the inability to readily conform to different facial features. This may not greatly affect printed face shields; however, it will likely have a significant effect on printed masks as said masks may fail fit tests when worn by different individuals. This nullifies the mechanical filtration of the printed mask to a degree as air will pass through the gaps between the mask and the face of the user and avoid the higher resistance filter. Additionally, this may make wearing a printed PPE uncomfortable for certain individuals. Hobbyists have recommended using flexible plastics, such as thermoplastic urethane (TPU) and thermoplastic elastomer (TPE), although these materials have an equally low glass transition temperature to PLA making them difficult to sterilize. Comfort foam inserts may be inserted into the posterior aspect of printed masks to accommodate different facial features. One innovative solution came in the form of the Bellus3D Mask Fitter, which involves scanning the face of the user through a mobile application and providing an STL file for a mask frame that is individually contoured and can be printed (Fig. 7) [24]. This will likely improve the seal of certain masks, as was evident in a study conducted in an ophthalmology setting during the COVID-19 pandemic. The study recruited 20 individuals who used a level 3 fluid resistant face mask with a 3D printed mask fitter on top in an effort to simulate N95 respirators. 90% of the participants (18 out of 20) successfully passed the fit test with only minor complaints regarding ease of use and comfort. This study provided a case for how 3D printing can augment traditional PPE performance [25].

3.5. Health Considerations
FDM 3D printers carry varying levels of health risks for users depending primarily on the type of plastic filaments utilized. Acrylonitrile butadiene styrene (ABS) plastics have been well documented to release larger amounts of Volatile Organic Compound (VOC) thermal decomposition products during printing when compared to PLA and other filaments. In total, nearly 216 VOCs were identified, many of which are carcinogenic. Reports also indicate a 33%-38% increase in airborne particle concentration after printing with ABS compared to PLA [26]. Regardless of the filaments used, almost all 3D printers release ultrafine particles (UFP), which pose a health risk to those chronically exposed. UFPs pose an increased risk for respiratory complications compared to fine particles as they are smaller in size and possess a larger surface area. As such, if UFPs are present in the lungs, they remain longer and tend to cause increased levels of inflammation, which manifest as cough and deteriorating shortness of breath [27]. Several studies have documented the onset of asthma in users heavily exposed to 3D printers. One such study surveyed multiple Canada-based companies, whose workers utilized 3D printing heavily as part of their normal workflow. 46 workers were surveyed in total, 27 of which complained of respiratory symptoms at least once weekly in the past year. The researchers concluded that exposure to operating 3D printers for over 40 hours weekly statistically significantly increased the likelihood of suffering from respiratory-related diseases, such as asthma and allergic rhinitis [28]. This is especially of interest in the context of PPE production during the COVID-19 pandemic, as many unexperienced individuals may obtain and run 3D printers with no hindsight of such potential health implications. Individuals wishing to print and distribute copious amounts of PPE may not have adequate safety equipment and work settings. It is therefore recommended to decrease exposure whenever possible to operating 3D printers and provide adequate ventilation and protective equipment to those interacting with the printers. Worth noting is that most studies conducted analyse the harms of 3D printing during the active process of printing, which involves melting plastics and releasing hazardous fumes and particles. However, another less researched area of interest is the possible biocompatibility issues of using 3D printed plastics as PPE. Biocompatibility depends on a host of factors, including duration and type of contact [29], as well as the nature of any trace materials in the printed product. One study conducted in 2021 investigated 24 traditional surgical and KN95 masks for toxic substances. Most of the masks tested contained trace elements below their corresponding detection thresholds; however, selected number of masks contained detectable amounts of trace elements. Examples of elements found were Pb, Cu, Zn, and Sb [30]. These potentially hazardous elements could be transferred to the user via a process called metal leaching, wherein the metals would dissolute from their natural ores into a liquid medium [31]. In this case, it is likely that leaching would be triggered by exposure to human saliva, such as in the case of prolonged mask usage, chewing on the mask, or violent sneezing. In the case of 3D printed PPE, selecting specific plastics may not entirely assure suitable biocompatibility as the process of additive manufacturing may modify the properties of said plastic used [32]. There has yet to be any formal testing for trace elements in 3D printed masks and other PPE. Conducting such a test in the future could potentially aid in identifying trace elements in printed products and their sources, allowing to avoid exposure to such elements.
CONCLUSION
While there are still some considerations that need to be addressed to ensure the safe and efficacious use of printed PPE, there is no doubt that many hospitals and communities alike have benefited from consuming 3D printed masks and face shields, particularly during the beginning of the pandemic. Additive manufacturing has aided in fulfilling the PPE demands during times of shortage, wherein tens of millions of additively manufactured face shields and hundreds of thousands of respirator and mask components were produced and distributed. While traditional PPE stocks have since recovered, it is of paramount importance to continue research and development into 3D printed PPE in order to create safer and more efficacious equipment that can equally match, or perhaps even supersede, traditional PPE. Some organizations, such as America Makes, have recommended further testing and validation of printed PPE in order to create a digital stockpile of vetted designs, which will be available on standby in cases of any future emergency circumstances. Documenting all of the aforementioned benefits and highlighting some possible drawbacks of this form of PPE production carry great significance considering the ongoing COVID-19 pandemic, as well as in case of any future public health emergencies.
LIST OF ABBREVIATIONS
| ABS | = Acrylonitrile Butadiene Styrene |
| FDA | = Food and Drug Administration |
| FDM | = Fused Deposition Modelling |
| NIH | = National Institute of Health |
| PLA | = Polylactic Acid |
| PPE | = Personal Protective Equipment |
| SEM | = Scanning Electron Microscope |
| SLS | = Selective Laser Sintering |
| STL | = Standard Tessellation Files |
| TPE | = Thermoplastic Elastomer |
| TPU | = Thermoplastic Urethane |
| UFP | = Ultra Fine Particles |
| VA | = Veterans Affairs |
| VOC | = Volatile Organic Compounds |
| WHO | = World Health Organization |
AUTHORS’ CONTRIBUTIONS
AA participated in developing the study concept and design, and drafted the article. BA also participated in developing the study concept and design, and substantively reviewed and modified the drafted article. All authors read and approved the final manuscript.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest financial or otherwise.
ACKNOWLEDGMENTS
Declared None.


