All published articles of this journal are available on ScienceDirect.
What's the Relation between Iron Deficiency (ID) and Febrile Seizure (FS)? A Case Control Study in Tehran, Iran
Abstract
Objective:
To evaluate the role of ID in the pathogenesis of FS.
Methods:
In this case-control study (2014-2016), 70 children were studied, 35 children with FS and 35 (controls)children with febrile diseases without convulsion (The mean age of cases was 2.191 ± 0946 vs. 1.93 ± 1.433 years in controls). Serum ferritin was estimated by the EIAS test. Data were compared between 2 groups, The ROC (receiver-operating-characteristic) curve was illustrated. The sensitivity, specificity, PPV, and NPV of the test, were calculated.
Results:
Serum ferritin levels had no significant difference between the 2 groups. The ferritin level (36ng/ml) had 74.3% sensitivity, 20% specificity, 56% PPV, and 52% NPV, with a Positive likelihood Ratio being 1.3 and a Negative likelihood Ratio: 0.93 to discriminate the 2 groups.
Conclusion:
Here the ferritin level (cut-off=36ng/ml) has an acceptable sensitivity (74.3%) but poor specificity (20%) and just 56% PPV and 52% NPV to differentiate the FS cases from non-convulsive febrile children. Although a different cut-off value 21.50 ng/ml provides 91% sensitivity and very low specificity . This lower threshold cut-off might have clinically relevant outcomes in FS children if considering the other comorbidities. In our opinion, ID could not lead to FS in all children, but in some cases, with a genetic basis; ID raises the threshold for seizures. The ferritin levels as an acute phase reactant are acceptable in every febrile case. The ferritin base level in each child (case /control) before infection was unknown, but in the present study, both groups were febrile in contrast to previous studies in which ferritin levels were compared with afebrile children. Due to the high prevalence of ID (26%), especially in the young Iranian population, adding iron to the diet might help decrease FS in susceptible cases. We recommend in the future study the FS cases selected with known iron levels before convulsion.
1. INTRODUCTION
Seizure disorders identify by episodes of abnormal, synchronous neural activity with cortical origin in the brain. These discharges produce abnormal electrical activity on EEG (1) When 2 episodes of seizure in 24 h happened in a patient Epilepsy is diagnosed [1, 2]. Lüders, et al (1999) classified seizures according to clinical history, neurological exam, ictal semiology and EEG, anatomical and functional neuroimaging findings. Seizures might be clinical (like as partial or generalized) or be obscure and subclinical [1]. Seizure disorders in older children (> 6 years) are similar to those in adults but is different in younger one [3-6] Seizure semiology in infants and children discussed by authors [3, 4] The incidence of unprovoked seizures and occurrence of neurodevelopmental delay reported by Andell et al Berg et al [5] revised terminology of seizures and epilepsies (2010). The International League Against Epilepsy (ILAE) revised the practical classification and Terminology of the seizure (2017) based on the 1981 Classification (extended in 2010) [7, 8]. This classification of epileptic seizures should be complemented by an epileptic syndrome classification to avoid the current confusion between the classification of epileptic seizures (electroclinical complexes) and the classification of epileptic syndromes [5].
Ishtiyaq et al, reported the incidence of seizure in the first year of life in Indian children [9] According to Ishtiyaq et al, 80% of cases presented with generalized tonic seizures, just 4% of cases had focal seizures and 14% was unclassified. Seizure recurrence and developmental delay were also studied during the first six months post [9]. Yang et al defined the seizure prediction model in critically ill Children [10].
One of the most common childhood convulsive disorders is FS, with a wide incidence rate in the world: 2–4% in the United States; 9-10% in and Japan, the highest rate being 14% in Guam [11-14) According to Fishman et al, FS is a seizure associated with a febrile illness in the absence of CNS infections or acute electrolyte abnormalities in 6-60 months old children without previous afebrile seizures [11],
Multiple etiologic factors are considered for FS such as:molecular genetic basis [12, 13], reduction of neurotransmitters (zinc and magnesium, GABA) [14-16] increased the pro-inflammatory cytokines; prostaglandins in sera or CSF of FS cases reported by Tutuncuoglu et al [17] ID reduces the metabolism of some neurotransmitters. On the other hand, fever may aggravate the negative effects of ID on the brain [18, 19] Pisacane et al. [19] showed a higher rate of ID in younger (<2yold) FS cases. Also, Kumar and Rajwanti and Al Rahman showed lower levels of ferritin in FS cases in comparison with non-convulsive febrile children [20-22].
Convulsive disorders especially FS are common causes of hospitalization in Iranian children (between 5 months to 6 years of age) [23-28].
Etiology, risk factors and outcome in refractory convulsive status epileptics in Iranian children discussed by Barzegae et al. [23]. The incidence rate of FS is 2-5% in our country [24-26].
In developing countries 46–66% of children under 4 years are anemic, with half attributed To ID [29, 30] according to a recent population-based study by Akbari et al, the prevalence rate of ID is 26.9% in the Iranian population and 14% in the young population (<18 years) [29] Nazari et al. (2018) study determined that about one-fifth of young Iranian children (<6 years old) suffer from ID anemia [30].
Recently, the relationship between ID and FC in Iranian children was reported by some authors. (Inconsistency and conflict were detected in the results of previous studies regarding the relationship between ID and FS [31-37]
The purpose of this prospective case-control study was to determine the relationship between ID and FS in our hospitalized children. Here, we report the ferritin cut-off level which could discriminate the FS cases from non-convulsive febrile children.
2. MATERIALS AND METHODS
This prospective case/control study was conducted for 2 years (2014-2016) in pediatric wards in Hazrat Rasoul (3rd level referral hospital) in Tehran, Iran.
This study was approved by the scientific advisory and ethical committees of Iran University of Medical Sciences. Written informed consent forms were signed by the Parents and the procedures involved complied with the Declaration of Helsinki.
Seventy febrile children were selected from cases who were admitted to the pediatric ward for 2 years A checklist was completed for the subjects who included complete history such as age, sex, seizure history, seizure type, and systemic diseases. One ml of blood in 2 groups in the first admission was tested for CBC, The serum ferritin for the cases and controls were studied by ELISA (enzyme-linked immunosorbent assay) on the first day of admission. These values are enrolled in the questionnaire.
2.1. Cases Definition
2.1.1. Inclusion Criteria
Thirty-five patients with the final diagnosis of FS (without underlying disease and normal CSF analysis) were selected as cases.
All febrile seizure group had the first bout of a single generalized febrile seizure prolonged <15 minutes.
2.1.2. Exclusion Criteria
All children with known causes for convulsion after complete clinical studies (e.g. electrolyte imbalance, leukemia, metabolic disorders, chronic diseases, malnutrition); CNS infection (meningitis, encephalitis, brain abscess) were excluded from the study. Also, we excluded all Children who had mental retardation, brain anomalies, brain tumor, atypical convulsion, focal seizure, chronic diseases, moderate to severe malnutrition,
2.2. Controls
Thirty-five febrile children without convulsion and normal CNS examination (unconsciousness r/ no meningeal signs and symptoms / no other abnormal neurologic deficits), negative bacterial culture (CSF/Blood). Viral infection was the final diagnosis febrile control group.
2.3. Lab Test
Two ml of peripheral blood was collected on the first day of admission to the hospital. The blood was tested for CBC, HB, MCV, MCH, MCHC in2 groups. The remaining blood in an acid-propylene tube was centrifuged and serum was preserved at -80°C. Serum ferritin was estimated by EIAS test for the cases and controls
2.4. Statistical Analysis
All analyses were conducted using SPSS, version 13.5. Quantitative variables were summarized as mean ±standard deviation (SD) and qualitative variables were counted and expressed as percentages. The Student’s t-test was used to determine significant differences in means of all continuous variables. Chi–square test was performed to compare the proportion between 2 or more discrete variables. P<0.05 was considered statistically significant.
ROC (receiver-operating-characteristic curve) was illustrated and the cut-off level for serum Ferritin, Sensitivity, specificity, PPV, NPV, of the test was also calculated.
3. RESULTS
Of 70 admitted children, the cases included 35 children (mean age: 2.191 ± 0946 years); 62.9% male,37.1% female who were referred due to FS. Also, 35 children who were hospitalized due to febrile diseases without convulsion enrolled in this trial as controls (mean age: 1.93 ± 1.433 years) 65.7% male, 34.3% female.
No significant difference was observed in age and gender between FC cases and controls (p value=0.3; 08). No significant difference was observed between 2 groups for mean Hemoglobin level (11.6 ± 079 vs. 11.86± 071; p value= 0.2); MCV (75.8 ± 4.3 vs. 77.62± 4.1, p value= 0.08); and MCH (26.2 ± 1.5 vs. 27.3± 2.8, p value= 0.07)
The mean level for serum ferritin in the 2 groups was 56.4ng/ml. All data Compared between cases and controls in Table 1.
Fig. (1) shows the area under the curve (AUC). AUC was 0.442(0.306-0.578, P value=0.4). According to this curve, the calculated cut-off level for ferritin was 36ng/ml.; it had 74.3% sensitivity, 20% specificity, 56% PPV, 52% NPV. The Positive likelihood Ratio: 1.3; Negative likelihood Ratio: 0.93 was calculated.
| Data | Hb | MCV /MCH | Ferritin level (ng/ml) | |||
| Case | Control | Case | Control | Case | Control ferritin | |
| Mean | 11.6 | 11.86± | 75.8 /26.2 | 77.62/27.3 | 54.57 | 58.31 |
| Standard deviation | 79 | 71 | 4.3 /1.5 | 4.1/2.1 | 24 | 23 |
| P value | 0.2 | 0.08 | 0.64 | |||
| Ferritin level (<36ng/ml) | ||
|---|---|---|
| Febrile Seizure | Positive | Negative |
| Positive | 9 | 26 |
| Negative | 7 | 28 |
The Cut off for ferritin (<36ng/ml) observed in 30% (9/35) of convulsive cases in comparison with 21% (7/35) in controls, without significant difference (54.57 ± 24 vs. 58.31± 23, p value= 0.64) (Table 2)
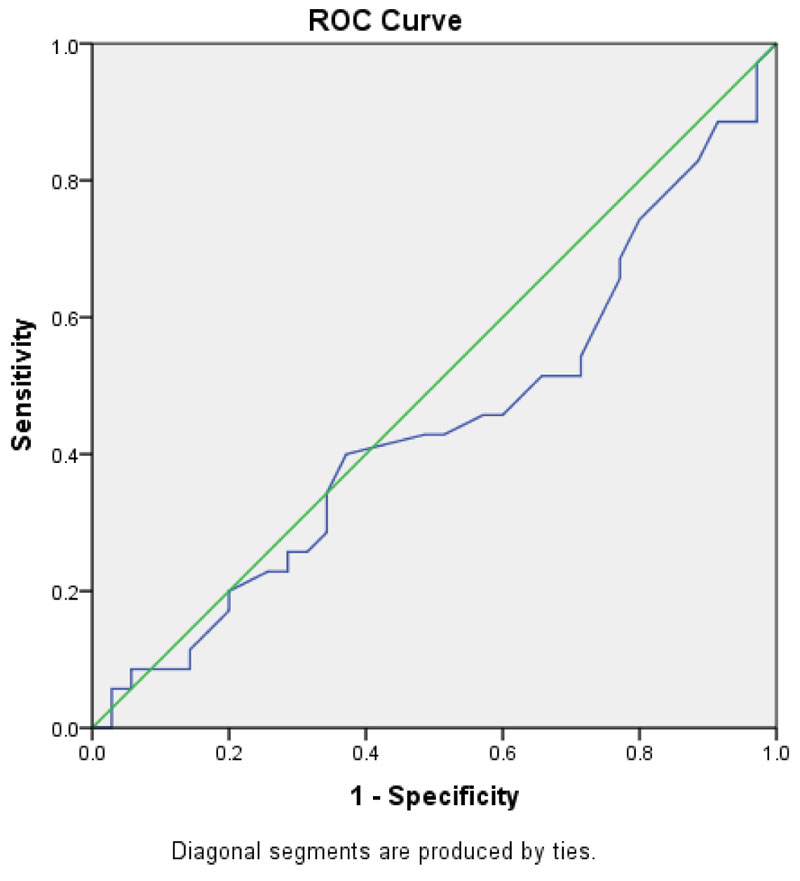
4. DISCUSSION
The present study is the first to report the ferritin cut-off level which discriminates the FC cases from non-convulsive febrile children. Ferritin cut off (36ng/ml) observed in 30% of FC cases without significant difference observed in (21%) of in non-convulsive febrile controls (p value= 0.64). This level has an acceptable sensitivity (74.3%) but poor specificity (20%) and just 56% PPV, 52% NPV to differentiate the FC cases from non-convulsive febrile children. The mean ferritin level (58 ng/ml) in all children in the present study is very close to normal children (58.75 ng/ml), as reported by Nazari et al [30]. The ferritin as an acute phase reactant in all febrile patients might explain this no significant difference.
Risk factors for recurrence of FS discussed by Moayedi et al. [25] Mahyar, Heydarian et al reported some risk factors for FS in Iranian children [26, 27]. Veisani et studied the familial History and recurrence of FS in a systematic review and Meta-Analysis [28].
The relationship between ID and FS was studied in Iran with contradictory results [31-37]. Some authors reported that ID was more frequent in children with FS in compare with non-convulsive febrile and normal children [31, 32]. On the contrary, Salehi, Talebian, [REMOVED HYPERLINK FIELD]showed that ID plays no role in pediatric FS[(33,34] Sharif et al reported ID in 45% of FS and 12% of non-convulsive febrile children [31] The 21% ID in non-convulsive febrile children (control group) in the present study was just similar to 26% study by Hashemi et al (26%). They realized that Children with FS were more likely to suffer from iron deficiency compared to those with solitary febrile illness or healthy children. Thus, ID could be considered a risk factor for FS, but in both mentioned studies, no cut-off point was determined for discrimination in three groups. ID could be an important risk factor for the development of FS. Evaluation of iron status was encouraged to be performed in children with FS. Indeed, Sadeghzadeh et al. [32] reported ID in 6% of FC cases in Zanjan; like as healthy groups. According to this study, it was suggested that although ID was not frequent in the febrile seizure group of children, iron deficiency was more reported in patients with fever [23]. The peak incidence for FS is near 18 months of age [2, 3].
Salehi et al. [33] observed that ID plays no role in pediatric FS. Talebian reported the pooled recurrent rate of febrile seizure in Iran was 20.9%, with positive family history in 28.8% (of children. They concluded that the risk of FS occurrence in children with ID seems to be more frequent than in children with convulsive febrile seizures, ID could be considered as a protective factor against the risk of convulsion occurrence by raising the threshold of convulsion [21, 34].
According to Fallah et al. [37] ID was more frequent in FS cases (48% vs. 28%, p= 0.04).In contrast, Bidabadi et al [36] reported that ID in FS was less than afebrile controls (44% vs. 48%) [36]. In the last decade, Nazari et al reported that ID is more prevalent in about one-fifth of Iranian children less than 6 years [30] Despite the efforts of the ministry of health and medical education of Iran for supplemental iron for all Iranian infants at least for 1–2 years; and also for girls, the prevalence of ID is considerable [29, 30].
The power of the present study is comparing the febrile children as controls, not afebrile children. Like us, Ghasemi et al reported ferritin levels (36ng/ml) in 40% of FS, 26% of children with non-convulsive febrile cases, and 12% of healthy children (non-febrile, non-convulsive) [35]. The cut-off level was not reached in any of the previous studies in Iran, except for the present study. In our study, all patients that were chosen as a patient group were admitted with their first FS, but in most previously mentioned studies, some FS cases had a recent history of febrile convulsion. Various diagnostic criteria of ID in different references and ferritin level as an age-dependent factor in the diagnosis of ID might be another reason for unmatched results [32, 33, 35]. We are in favor of the results mentioned in Bidabadi et al. [36] study that no identified relationship has been detected yet between ID and FS, maybe it could be considered as an accidental correlation or interference of other unknown factors such as genetic, viral and other transmitters [36].
CONCLUSION
The present study reports the ferritin level (cut-off=36ng/ml) has an acceptable sensitivity (74.3%) but poor specificity (20%) and just 56% PPV and 52% NPV to differentiate the FS cases from non-convulsive febrile children. Although a different cut-off value 21.50, ng/ml provides 91% sensitivity and very low specificity. This lower threshold cut-off might have clinically relevant outcomes in FS children if considering the other comorbidities. In our opinion, ID could not lead to FS in all children, but in some cases, with a genetic basis; ID raises the threshold for seizures. The ferritin levels as an acute phase reactant are acceptable in every febrile case. The ferritin base level in each child (case /control) before infection was unknown, but in the present study, both groups were febrile in contrast to previous studies in which ferritin levels are compared with afebrile children. Due to the high prevalence of ID (26%), especially in the young Iranian population, adding iron to the diet might help decrease FS in susceptible cases. We recommend in the future study the FS cases selected with a known iron level before convulsion.
LIST OF ABBREVIATIONS
| GABA | = Gamma Aminobutyric acid |
| FS | = Febrile Seizure |
| ID | = Iron efficiency |
| CBC | = Complete Blood Cell |
| HB | = Hemoglobin |
| CSF | = Cerebro Spinal Fluid |
| CNS | = Central Nervous System |
| EIAS | = Enzyme- Immunoassay |
| ROC | = Receiver-Operating-Characteristic Curve |
| PPV | = Positive Predictive Value |
| NPV | = Negative Predictive Value |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the Ethical Committee at the Iran University of Medical Sciences. IR.IUMS.REC1394.32104.
HUMAN AND ANIMAL RIGHTS
No animals used that are the basis of this study. All the human procedures were performed in accordance with the 1975 Declaration of Helsinki.
CONSENT FOR PUBLICATION
The authors confirm that written informed consent has been taken from the patients /Volunteers/ guardians of the children for this study.
STANDARDS OF REPORTING
STROBE guidelines have been followed.
AVAILABILITY OF DATA AND MATERIALS
The authors confirm that the data supporting the findings of this study are available within the manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest in preparing this study.
FUNDING
This study was funded by the Iran University of Medical Sciences Faculty of Medicine.
ACKNOWLEDGEMENTS
Declared none.


