All published articles of this journal are available on ScienceDirect.
An Integrated Reporting Tool for Management of Hiv And Non-communicable Diseases for Primary Health Care Facilities in Limpopo Province, South Africa
Abstract
Introduction:
Reporting of a health programme such as integrated management of the Human Immunodeficiency Virus (HIV) and Non-communicable Diseases (NCDs) is essential for programme evaluation to improve patient clinical outcomes. It is a criterion that all services that are provided in primary health care facilities and hospitals are monitored, evaluated and reported according to the approved reporting tools and approved health indicators.
Aim:
This study aimed to develop a reporting tool to report the implementation of integrated management of HIV and NCDs in Limpopo Province, South Africa.
Methodology:
The South African National Indicators Data Sets (NIDS) were adopted and used to develop the integrated management of HIV and NCDs reporting tool. A Delphi technique was used to validate the developed reporting tool. Eight (8) HIV and NCDs programme experts participated in the review process to establish the validity of the developed tool.
Results:
Eight integrated HIV and NCDs data elements reporting tool were developed and reviewed by eight (8) programme experts. The tool was found to be relevant and useful and likely to be adopted by Limpopo province for implementation.
Conclusion:
This was the first integrated HIV and NCDs reporting tool to be developed for Nurse Initiated Management of Antiretroviral Therapy (NIMART) Nurses in Limpopo Province to serve as a basic reporting tool to improve the integrated management of HIV and NCDs including patient outcomes.
1. INTRODUCTION
The reporting of patient clinical outcomes has been the backbone for health programme implementation across the globe. The South African National Department of Health (NDoH) in terms of the National Health Act (NHA) (Act 61 of 2003) [1] stipulates that to facilitate and coordinate the establishment, implementation and maintenance of the information systems by provincial departments, district councils, municipalities, and the private health sector at national, provincial and local levels to create a comprehensive National Health Information system [1]. Therefore, the District Health Management Information System (DHMIS) [2] is used to track the health service delivery including integrated management of human immunodeficiency virus (HIV) and non-communicable diseases (NCDs) in districts. During service delivery, Nurse Initiated Management of Antiretroviral Therapy (NIMART) nurses are expected to report their health care activities to inform policymakers and to improve the quality of patient care [3].
In 1999, South Africa adopted and rolled out the national minimum data elements indicators or primary health care (PHC) and they were implemented in all public primary health facilities. During the rollout, the standardised PHC tick registers were given to PHC facility staff for use to report the services rendered to the patients and for proper planning and budgeting of health care services [4, 5]. Furthermore, reporting tools were added when a new health programme is introduced. In 2014, integrated management of chronic diseases programme was introduced with its reporting tools. However, the reporting tools were unilateral and not integrated [6]. It was therefore important for the researcher to develop some data elements to address the existing unilateral datasets.
From the literature reviewed, it has been documented that leveraging on existing HIV programmes can improve the reporting of integrated management of HIV and NCDs [8-12]. In addition, the current reporting tool for chronic diseases only reports the HIV and TB integration or collaboration. Table 1 outlines the datasets that are collected for chronic diseases in South Africa [2].
In Limpopo, there is no evidence of integrated management of HIV and NCDs reported as an integrated programme. NCDs are now considered to be a threat to many lives and South Africa is faced with the collusion of HIV and NCDs [13, 14]. Therefore, the reporting of how HIV and NCDs impact patients’ clinical outcomes is essential to preserve human life and to meet the sustainable development goal 4 [15]. It is paramount to note that this research focuses on HIV, diabetes and hypertension integration. Therefore, the purpose of this research was to develop a scientific reporting tool for the integrated management of HIV and NCDs in Limpopo Province, South Africa.
| NIDS for NCDs in South Africa |
| · Client 40 years and older screened for hypertension |
| · Hypertensions visit by clients on treatment |
| · Client 40 years and older screened for diabetes |
| · Diabetes client 40 years and older new |
| · Diabetes treatment visit |
| · Hypertension client 18-44 years new |
| · Hypertension client 45 years and older new |
| · Diabetes client 18-44 years new |
| · Diabetes client 45 years and older new |
1.1. Aim
This study aimed to develop a reporting tool for the implementation of integrated management of HIV and NCDs in Limpopo Province, South Africa.
| Reporting Tool Review Questions. | Q1 | Q2 | Q3 | Q4 | Comments |
| Respondent 1 | Yes | Yes | No | Likely | I have made some comments on the tool, which I would like to be considered. And would it be helpful to add metal illness like Depression or would that be expanding to another realm of chronic illnesses? |
| Respondent 2 | Yes | Yes | No | Likely | Hypertension/Diabetes patient tested HIV positive (with age categories) Hypertension / Diabetes patient initiated on ART Rationale for 1 - 2: To check if all patients were initiated on ART after testing HIV positive. To address the 90/90/90 strategy. |
| Respondent 3 | Yes | Yes | No | Likely | No comments |
| Respondent 4 | Yes | Yes | No | Likely | The tool is relevant for the integration of services; however, the ART clinic is no longer in use in Limpopo Hospitals as all clients have been down referred to the PHC facilities. Pick up point will be CCMDD to replace ART Clinic. There is also Fast lane que especially for the workers and students. The other source of information will be Tier.net I also think the tool must accommodate less than 18yrs or 18yrs and below for the children who are diagnosed with Diabetes at an early age. I also think the frequency must accommodate those that are stable and referred to CCMDD and are collecting treatment once in six months to decongest the facilities. |
| Respondent 5 | Yes | Yes | No | Somewhat likely | Feasibility regarding the use of the tool in the facility. Interviewing the facility manager-he/she can provide a better perspective regarding the tool as they are at operational level. Other comments insert inserted next to each area. |
| Respondent 6 | Yes | Yes | No | Likely | No comments |
| Respondent 7 | Yes | Yes | No | Likely | No comments |
| Respondent 8 | Yes | Yes | No | Likely | No comments |
| Total | 100% | 100% | 100% | 87.5% (n=7) & 12.5% (n-1) |
2. RESEARCH METHODOLOGY AND DESIGN
2.1. Study Design
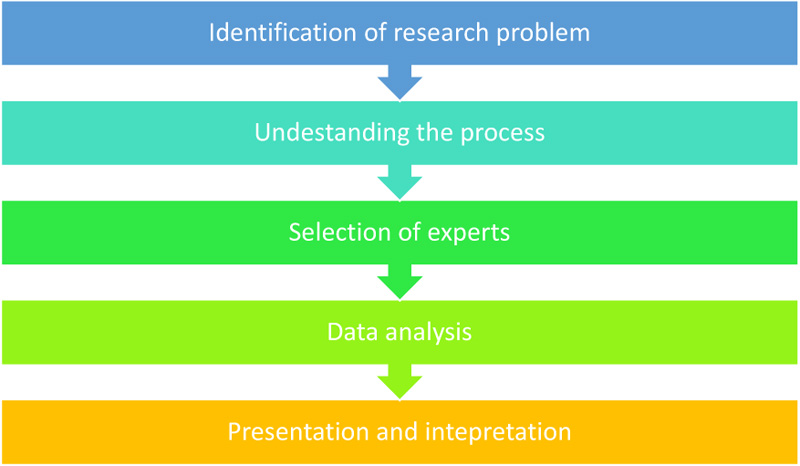
The researcher developed the reporting tool guided by the existing data elements as shown in Table 1, followed by a review by programme experts from both the public and private health sectors. A Delphi technique was employed with the use of programme expert review. The Delphi technique is a methodological and interactive research procedure for obtaining the opinion of a panel of independent experts concerning a specific subject [16]. This research method also suggests that the researcher collects and analyse available information regarding a specific topic for breadth and depth of understanding and corroboration [17, 18]. The researchers used the proposed research guidelines for using the Delphi technique as outlined by Hasson Felicity, Keeney and Mckenna [19]. The study followed the five processes of the Delphi technique, namely, Identification of research problem, Understanding the process, Selection of experts, Data analysis, Presentation and findings.
2.2. Methodology
A multistage process of Delphi was used to combine the responses of the experts and the findings of the qualitative and quantitative results [19, 20]. Fig. (1) depicts the process followed:
2.2.1. Step 1: Research Problem Identification
During problem identification, the researcher must centre upon the appropriateness and correlate available information regarding the topic [18, 21]. The research emanated from the PhD work in which the researchers were developing a conceptual model to strengthen the implementation of integrated management of HIV and NCDs among NIMART nurses working at PHC facilities where both the literature review, quantitative and qualitative results prompted the researchers to develop the reporting tool including the researcher’s personal experiences of over a decade working in public health facilities as a NIMART nurse across three provinces in South Africa. The researcher learnt that there is a dire need to integrate the management of HIV and NCDs in PHC level and there is a need for developing the integrated reporting tool. From the literature review, we have found that there is no reporting tool for integrated management of HIV and NCDs. However, there are separated reporting data tools for HIV and NCDs [10]. In addition, we have observed that there is an integration of communicable diseases, such as HIV and TB are reported together. However, hypertension and diabetes are reported separately as shown in Table 1. In addition, from the reviewed literature, Hypertension and Diabetes Mellitus were more managed by NIMART nurses in PHC facilities [4, 8, 19].The above elements facilitated the identification of the problem and the ability to draft the reporting tool for integrated management of HIV and NCDs.
2.2.2. Step 2: Understanding the Process
The Delphi method requires the researcher to understand the multistage processes involved to reach a consensus by using surveys and getting feedback whereby respondents may respond to the survey anonymously [18, 21]. In this study, the researchers developed the integrated reporting tool for the nominal group for them to give comments and provide feedback to reach a consensus. The reporting tool is shown in Table 2. In addition, the researcher had to gather all the information sent by the reviewers to finalise the reporting tool.
2.2.3. Step 3: Selection of Experts (HIV and NCDs Programme Experts)
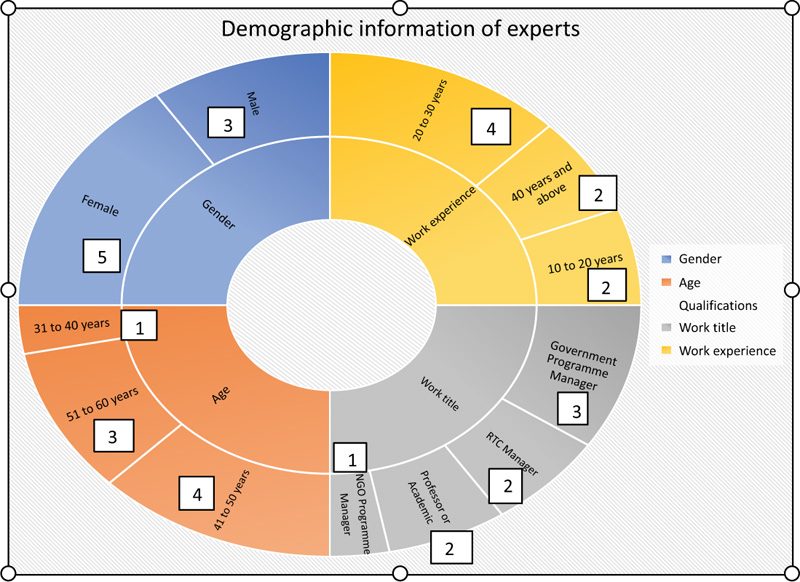
It is a requirement in a Delphi method to select experts of a particular topic or field. The number of experts may be 60 or maybe fewer than 15 [18, 21]. In this study, we selected experts from different fields, namely, the programme managers at the NDoH who were directly involved or developing HIV and NCDs policies and guidelines, the provincial managers who oversaw the implementation at provincial level, the regional training centre managers who were directly involved in the in-service of professional nurses on treatment guidelines, the programme managers from the donor-funded organisation and the university lecturers who were responsible for communicable and NCD programmes. In addition, the Delphi technique is useful for reaching consensus in health research and priority health programmes [23]. We used the experts who were directly involved in HIV and NCDs in different sectors to get a broader perspective on the selected topic. (Fig. 2) shows the demographic information of the experts who participated in the study. A total of eight (8) experts participated in the reporting tool review.
2.2.4. Informing Experts
In this step, it is important to explain what is required of them, how much time it will require, what they will be required to provide, what the purpose of the study is to be, and what will be done with the information [18, 21]. A letter to participate in the review and the instructions were sent to the programme managers identified above through emails. The letter requested the experts to participate in the reporting tool review. After receiving the confirmation to participate, an instruction letter stating what is expected of them was sent again. In addition, the reporting tool was attached for experts to review for reference. A total of 27 emails were sent out to the experts identified, the number of sent emails was informed by the number of experts who were identified during HIV and NCD review meetings and conferences where the researcher was participating. The researcher received the agreement to participate from 15 programme experts. However, only eight participated in the review. A period of two weeks (14 days) was given to the experts to send back their responses. The instruction letter to the programme experts included the following 1) Section A demographic information, and 2) Section B reporting tool review questions. Question 1 was related to the relevance of the reporting tool, question 2 was related to the usefulness of the tool, question 3 was asking if the tool is not required to monitor integrated management of HIV and NCDs and question 4 was asking the likelihood of the developed reporting tool to be adopted by the NIDS committee.
2.2.5. Step 4: Data analysis
In this step, the researcher was required to analyse the data received from the programme experts. The experts shared their opinions by completing the instruction sheet. Important issues were analysed by using the excel spreadsheet to reach a consensus on the information gathered from experts. The researcher gathered all the responses. Furthermore, the researcher managed and analysed all responses. It is suggested that 80% of similar responses are adequate to reach a consensus on the topic being studied [21]. The results of the first round indicated a reasonably tight grouping of opinions on most of the questions. In round two, each expert was given their answers to the first round of questions. Then the experts were asked to reassess their first session answers and to change them if they think they answered wrongly in the first round. Only two rounds were necessary as the responses on the second round were the same as those of the first round. Table 2 shows the responses from the respondents. All eight programme experts indicated that the reporting tool is relevant and useful for monitoring the integrated management of HIV and NCDs in PHC facilities which showed 100% of similar responses. For the question which states that the reporting tool is not required for monitoring and evaluation of integrated management of HIV and NCDs, all eight respondents indicated a ‘No’. The last question needed respondents to indicate how likely the reporting tool will be adopted for implementation in the district. Seven experts indicated that the reporting tool is likely to be adopted and one expert indicated that it is somewhat likely to be adopted by the NIDS committee. The panel members were also provided with the opportunity to provide comments to improve the reporting tool. As shown in Table 2, five experts commented on the tool, the other three experts did not comment. Two respondents alluded to the relevance of the reporting tool. Two respondents alluded to the spelling and typo errors in the reporting tool through the inserted comments. Two experts indicated that the reporting tool should indicate the age categories for hypertension and Diabetes Mellitus as it will assist in checking whether all patients were initiated on ART after testing positive for HIV. Furthermore, one of the experts would like the reporting tool to include TB. The experts also commented on adding mental health conditions to the reporting tool. There were some outlying comments by two experts which were not related to the reporting tool review as indicated in respondents 4 and 5. Three experts did not comment on the reporting tool.
2.2.6. Step 5: Presentation and interpretation
The last step in the Delphi technique is to present and interpret the findings through statistics, graphs and narration [16-18, 21]. For this step, the researcher acted on the comments made by the experts to finalise the reporting tool. The researcher added the age categories as suggested by the experts. Furthermore, the reporting tool was sent to the language editor for editing purposes. The addition of mental health conditions and TB could not be added as the aim of developing the reporting tool was focused on HIV, hypertension and diabetes, however, the addition of the suggested health conditions can be incorporated in another study. Table 3 presents the final integrated reporting tool for integrated management of HIV and NCDs for Limpopo Province. The reporting tool can be included in the current PHC tick register. The reporting tool is copyrighted and is accessible through request from author. Table 4 provides the definition of the developed data elements, the rationale for collecting the developed data elements, the collection point of the developed data elements, the person responsible for collecting data, the collection point, the collection tools which can be used to collect the developed data elements and the frequency of collecting data.
Facility name……………………………Month……………………………………Consulting room no…………………………….
Professional Name……………………..Signature…………………Reviewed by………………….Signature and date……………
(Authors own work).
| Date | Patient NO | Folder NO | Name | Gender | Age | Integrated Management of HIV and NCDs | |||||||||||
| Diabetes and ART | Hypertension and ART | ||||||||||||||||
| ART patient 18 years and below diagnosed with diabetes | ART 18-44 years diagnosed with diabetes | Diabetes Mellitus clients 18-44 years and older new screened for HIV | Diabetes Mellitus clients 45 years and older new screened for HIV | Diabetes Mellitus adult 18-44 years on diabetes treatment at the start of ART | Diabetes Mellitus adult 45 years old and older on diabetes treatment at the start of ART | ART patient 18 years and below diagnosed with hypertension | ART 18-44 years diagnosed with hypertension | ART 18-44 years diagnosed with hypertension | Hypertension client 45 years and older new screened for HIV | Hypertension client 18-44 years on hypertension treatment at the start of ART | Hypertension client 45 years old and older on hypertension treatment at the start of ART | ||||||
| Running Total | |||||||||||||||||
| 1 | |||||||||||||||||
| 2 | |||||||||||||||||
| 3 | |||||||||||||||||
| 4 | |||||||||||||||||
| 5 | |||||||||||||||||
| 6 | |||||||||||||||||
| 7 | |||||||||||||||||
| 8 | |||||||||||||||||
| 9 | |||||||||||||||||
| 10 | |||||||||||||||||
| 11 | |||||||||||||||||
| Data Element | Definition | Rationale | Collected by | Collection Point | Tools | Frequency of Reporting |
| ART patient 18-years-old and below diagnosed with diabetes | This will mean an HIV positive patient 18 years and below who is diagnosed with Diabetes Mellitus | To monitor HIV/diabetes management of patient on ART | NIMART Nurse | PHC Clinic CHC ART Clinic |
PHC Tick Register Adult patient stationery |
Monthly |
| ART 18-44 years old diagnosed with diabetes | This will mean an 18–44-years-old patient already on ART then developed or diagnosed with diabetes while on ART | To monitor HIV/diabetes management of patient on ART | NIMART Nurse | PHC Clinic CHC ART Clinic |
PHC Tick Register Adult patient stationery |
Monthly |
| Diabetes Mellitus clients 18–44-years-old new screened for HIV | This will mean an 18–44-years-old diabetic patient who is diagnosed in the facility using appropriate clinical guidelines and have been screened and tested for HIV | To monitor the screening of HIV among patients newly diagnosed with hypertension | NIMART Nurse | PHC Clinic CHC ART Clinic |
PHC Tick Register Adult patient stationery |
Monthly |
| Diabetes Mellitus clients 45-years-old and older new screened for HIV | This will mean 45-years-old and older diabetic patient who is diagnosed in the facility using appropriate clinical guidelines and have been screened and tested for HIV | To monitor the screening of HIV among patients newly diagnosed with hypertension | NIMART Nurse | PHC Clinic CHC ART Clinic |
PHC Tick Register Adult patient stationery |
Monthly |
| Diabetes Mellitus adult 18-44 years on diabetes treatment at the start of ART | This will mean an 18–44-years-old patient who were on diabetic treatment at the start of ART | To monitor HIV/diabetes management of patient on diabetes treatment | NIMART Nurse | PHC Clinic CHC ART Clinic |
PHC Tick Register Adult patient stationery |
Monthly |
| Diabetes Mellitus adult 45-years-old and older on diabetes treatment at the start of ART | This will mean a 45-years-old and older patient who were on diabetic treatment at the start of ART | To monitor HIV/diabetes management of patient on diabetes treatment | NIMART Nurse | PHC Clinic CHC ART Clinic |
PHC Tick Register Adult patient stationery |
Monthly |
| ART patient 18-years-old and below diagnosed with hypertension | This will mean an 18-years-old and below patient already on ART then develops or diagnosed Hypertension while on ART | To monitor HIV/hypertension management of patient on ART | NIMART Nurse | PHC Clinic CHC ART Clinic |
PHC Tick Register Adult patient stationery |
Monthly |
| ART 18-44 years diagnosed with hypertension | This will mean an 18-44-years-old ART client newly with hypertension diagnosed in the PHC facility using appropriate clinical guideline. | To monitor HIV/hypertension management of patient on ART | NIMART Nurse APC Nurse |
PHC Clinic CHC ART Clinic |
PHC Tick Register Adult patient stationery |
Monthly |
| Hypertension client 18–44-years-old new screened for HIV | This will mean an 18–44-years-old hypertension client newly diagnosed in the PHC facility using appropriate clinical guidelines and have screened or tested for HIV | To monitor the screening of HIV among patients newly diagnosed with hypertension | NIMART Nurse | PHC Clinic CHC ART Clinic |
PHC Tick Register Adult patient stationery |
Monthly |
| Hypertension client 45 years and older new screened for HIV | This will mean a 45-years-old and older hypertension client newly diagnosed in the PHC facility using appropriate clinical guidelines and have screened or tested for HIV | To monitor the screening of HIV among patients newly diagnosed with hypertension | NIMART Nurse | PHC Clinic CHC ART Clinic |
PHC Tick Register Adult patient stationery |
Monthly |
| Hypertension client 18–44-years-old on hypertension treatment at the start of ART | This will mean an 18–44-years-old patient who were on hypertension treatment at the start of ART | To monitor HIV/hypertension management of patient on hypertension treatment | NIMART Nurse | PHC Clinic CHC ART Clinic |
PHC Tick Register Adult patient stationery |
Monthly |
| Hypertension client 45-years-old and older on hypertension treatment at the start of ART | This will mean a 45-years-old and older patient who were on hypertension treatment at the start of ART | To monitor HIV/hypertension management of patient on hypertension treatment | NIMART Nurse | PHC Clinic CHC ART Clinic |
PHC Tick Register Adult patient stationery |
Monthly |
3. RESULTS AND DISCUSSION
The Delphi technique was employed in the review and validation of the integrated reporting for HIV and NCDs as it was very suitable for the researcher to obtain the insights of programme experts. In this study, the experts shared their views and comments regarding the developed reporting tool and the researcher heeded to their comments. The Delphi method produced the results which assisted the researcher to modify the reporting tool. Most researchers [22-27] who used the Delphi technique alluded that it investigates complex and multidimensional topics. After two rounds of responses, the researcher was able to finalise the reporting tool which was said it was most likely to be adopted by the NIDS committee in South Africa and was relevant and useful for monitoring and evaluation of the implementation of integrated management of HIV and NCDs in Limpopo Province.
The reporting tool includes the data elements which integrate HIV, Diabetes Mellitus, HIV and hypertension. The researcher collated the comments given by the programme experts. The following narration shows how the comments were attended to by the researcher. However, some comments were not effected as the study was focussed mainly HIV, Diabetes Mellitus and Hypertension.
- Age categories – the researcher included the age categories of less than 18 years and 45 years and above.
- Screening of HIV positive patients for Diabetes Mellitus and hypertension – the researchers added the screening of both conditions in the reporting tool.
- Inclusion of mental health – this comment was not effected as the study focused on diabetes and hypertension.
- ART clinic be replaced with CCMDD – this was not changed as the Central Chronic Medicine Dispensing and Distribution (CCMDD) is a medicine collection point by patients.
- Interviewing of facility managers regarding the use of the tool – this was noted and maybe considered in future research where the tool is will be implemented.
- Comments inserted in the tool itself – the comments inserted on the reporting tool were related to spelling of words where the researcher has corrected the spelling of words on the proposed tool.
According to different authors who have used the Delphi method, experts provide feedback according to their understanding of the programme being reviewed [23-25]. In this study, the HIV programme experts have provided relevant inputs when reviewing the reporting tool. However, there were some experts who provided the comments based on the broader understanding of the chronic conditions. After considering the experts comments, the final integrated reporting tool for HIV, diabetes and hypertension showed more strength where it can be adopted and used in different PHCs in the country.
3.1. Limitations
The limitation of this study was the non-response by other experts which may have brought many comments on the reporting tool or important issues not reflected in addition the sample size comprised of eight participants and focussed in one province which affect generalisation. However, the experts were selected from different department levels which allow the tool to be implemented in all provinces of South Africa. Another limitation is the focus on two NCDs (Diabetes Mellitus and Hypertension), however, this opened up the need to include other NCDs in future research.
CONCLUSION
The study aimed at developing the integrated reporting tool for HIV and NCDs management in Limpopo Province. The researchers developed the reporting tool and through the Delphi technique. We used various HIV and NCDs programme experts to review, validate and provide comments on the reporting tool. The developed and validated integrated reporting tool for HIV and NCDs may serve as a basis for the PHC data collection including the monitoring and evaluation team, and maybe added in the PHC tick registers for use by NIMART nurses providing integrated management of HIV and NCDs within the PHC context. The NDoH may adopt it and implement the reporting tool for PHCs in all provinces in South Africa. The reporting tool was found to be useful, relevant and it is likely that it can be implemented in Limpopo province and other provinces in South Africa.
LIST OF ABBREVIATIONS
| HIV | = Human Immunodeficiency Virus |
| NCDs | = Non-communicable Diseases |
AUTHORS’ CONTRIBUTIONS
NSMM, LM and LAS contributed to the conceptualisation of the study and design of the study. NSMM conducted the data collection and analysis. NSMM, LM and LAS wrote, edited and approved the final version of the manuscript for submission.
ETHICAL STATEMENT
The study was reviewed and approved by the North-West University (NWU), Limpopo Department of Health Research Committee. Consent was considered implied by participation in the study as approved Ethics number: NWU-0957-19-51.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data sets and materials are available from the corresponding author [N.S.M] on request.
FUNDING
The study was funded by the North-West University and the Health and Welfare SETA (HWSETA).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
We acknowledge the members of the National Department of Health, Regional Training Centre Managers, Academic and Non-Governmental Organisations members for their participation in the study.


