All published articles of this journal are available on ScienceDirect.
Antenatal Diagnosis of Congenital Heart Disease in the State of Alabama: Challenges and Opportunities
Abstract
Background:
Antenatal diagnosis of congenital heart disease (CHD) has positive effects on clinical outcomes. However, the prevalence of antenatal diagnosis remains low. The objective of this study is to measure the prevalence and distribution of antenatal CHD diagnosis in Alabama.
Methods:
Data were obtained from the Society of Thoracic Surgeons national database on surgeries for children with CHD and stratified by antenatal diagnosis. Demographic, census, and hospital data were compared between pre- and post-natally diagnosed cases. Cases were mapped by ZIP code to describe the distribution for the prevalence of CHD antenatal diagnosis.
Results:
From 2013-2019, 1733 children required cardiac repair for CHD, 20% were diagnosed prenatally and 80% postnatally. Only 43% of those with Hypoplastic Left Heart Syndrome, 22% with Tetralogy of Fallot and 26% with Transposition of the Great Arteries had a prenatal diagnosis. No factors were associated with receiving a prenatal diagnosis. Lastly, 82% of ZIP codes were below the reported national average for antenatal CHD diagnosis.
Conclusion:
Prenatal detection of CHD in Alabama is lower than the reported national averages. More studies are needed to explore reasons for missed antenatal CHD diagnoses. Mitigation of factors related to low antenatal diagnosis can support patients and improve neonatal outcomes.
1. INTRODUCTION
In the United States, congenital heart disease (CHD) occurs in almost 1% of births per year [1]. While many CHDs are mild in nature, one in four babies born with a CHD has a critical or severe CHD [2]. Severe cases must be treated soon after birth and may require special care, cardiac catheterization, cardiac surgery, or in some cases, heart transplantation. These interventions are limited to highly specialized hospitals [3-5]. Antenatal detection of CHD generally occurs during the anatomic ultrasound performed between 18 to 22 weeks gestation or during a fetal echocardiogram (Fig. 1) that is typical for patients with an abnormal anatomic ultrasound, family history of CHD, or maternal medical comorbidity with increased risk for CHD, such as diabetes [6, 7]. In the United States and other developed countries, despite the high accuracy of fetal echocardiographic methods, the antenatal CHD detection rate remains lower than 50% [8-10].
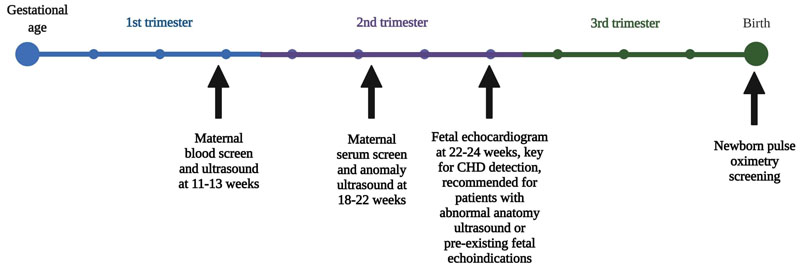
Structurally critical CHDs (CCHDs) constitute around 25% of overall CHDs and are important to detect before birth, as they require immediate care at birth and surgical repair [1]. The prevalence of antenatal diagnosis has been reported in various studies, and the detection rate can vary based on defect size. According to an analysis of data from the US Society of Thoracic Surgeons (STS), the reported prevalence of antenatal diagnoses for large defects or CCHD is 67.4% for hypoplastic left heart syndrome (HLHS), 26.6% for Tetralogy of Fallot (TOF), 41.3% for TOF with absent pulmonary valve, and 36.8% for transposition of the great arteries (TGA) with the ventricular septal defect [11]. A retrospective study has shown that antenatal diagnosis of CHD allows for planned delivery with proper multi-disciplinary care coordination [12]. Other studies have shown that antenatal diagnosis is associated with decreased odds of needing intubation and timely treatment with prostaglandins, reducing the risk of metabolic acidosis [13, 14].
The cost-benefit analysis of antenatal screening for cardiac anomalies has been controversial [15]. Given the variation in screening protocols, training, and accuracy, it has been debated that screening may not be worth the effort. However, postnatal diagnosis of CHD incurs disproportionate costs compared to antenatal diagnosis outcomes, and calculations of improved CHD antenatal detection rates clearly demonstrate significant clinical and financial benefits [16]. The effect that antenatal diagnosis of CCHD has on surgical outcomes is not clear. While many studies show no significant impact on pre- or post-operative mortality in the prenatal screening of CCHD, there have been positive associations reported between screening and pre-operative morbidity (i.e., antibiotic use, mechanical ventilation, hepatic dysfunction, renal dysfunction, and acidosis) [17, 18]. Few studies have examined the antenatal CHD diagnosis rate at a regional, provincial, and state level, which suggests that the larger scale may not reflect what is occurring in smaller geographic areas [11].
As of 2013, CHD screening was mandated via the Alabama Newborn Screening Program under the Alabama Department of Public Health [19]. Current guidelines for CCHD screening at the hospital include a pulse oximetry measurement after birth following the guidelines outlined by Kemper et al. [20]. An article published by Pediatrics and the CDC in 2015 reported that half of all infants born annually in the U.S. with severe CHDs that are detected late (on or after 3rd day after birth) would be missed by pulse oximetry screening for newborns [21]. This number accounted for around 14% of estimated CHD births per year, highlighting the need for continued CHD screening at all stages. Currently, in the state of Alabama, there are 2 level 4 Neonatal Intensive Care Units (NICU) (both in Birmingham, AL), and 9 level 3 NICUs that are generally relegated to one per region of the state [22]. With a low number of high-level NICUs in the state, failed pulse oximetry screenings require transport to a larger tertiary hospital. Cases of severe CHD requiring immediate surgery must be transported to a single location in the central area of the state, which is an additional challenge to receiving critical care for children born a long distance to a high-level NICU. Improved prenatal diagnosis can allow proactive planning to reduce these logistical issues.
Given the relatively low rate of CHD diagnosis overall (~30-50%), it is critical to understand this issue in the context of the southern U.S [15]. This study describes the prevalence and distribution of antenatal diagnosis for CHD in Alabama, a southern U.S. state that is rural and has high rates of poverty and adverse health outcomes. The results from this study may help inform health officials in the state and possibly serve as an example for other states to make a similar assessment in order to inform systems-level changes in maternal and child healthcare.
2. METHODS
After institutional review board approval (IRB-1106 26007), a query was performed using our site’s STS database for all children with CHD who had a surgical cardiac repair at the Children’s Hospital of Alabama between August 13th, 2013, and December 31st, 2019. The ethics board deemed this an exempt protocol and waived the need for consent due to the methodology of this study. Sociodemographic and clinical data were extracted either from STS or manually from electronic medical records. Distance to the clinic was calculated using the child’s ZIP (Zone Improvement Plan) code and mapping it to the nearest hospital/clinic with prenatal care services [23]. The STAT (The Society of Thoracic Surgeons-European Association for Cardio-Thoracic Surgery) variable, used to assess CHD severity, is a score that describes the mortality risk associated with a cardiac surgical repair on a scale from 1-5, with 5 meaning highest mortality risk. Genetic abnormality was defined by the STS database as having any non-cardiac structural abnormality or genetic syndrome. Median income by ZIP code was obtained from the inflation-adjusted income statistics from the American Community Survey available from the US Census Bureau (Table ID S1901) for the year the patient had surgery. Rural and urban status of patients’ ZIP codes was based on the United States Department of Agriculture’s Rural-Urban Commuting Area classification from the 2010 census. The primary outcome was the presence of an antenatal CHD diagnosis, as present in the electronic medical record reported by the parent upon admission to their surgical encounter.
Data related to CHD antenatal diagnosis status were described and compared using Chi-square, Fisher’s exact test, Wilcoxon rank sum and student t-test where appropriate. Regression analysis was then performed to identify possible variables associated with not receiving an antenatal CHD diagnosis. A secondary analysis was performed only among certain CCHDs (HLHS, TOF and TGA) to analyze mortality and re-operation differences by antenatal diagnosis status. Statistical Analytical Software 9.4 (SAS institute, Cary, NC) was used for the analysis and all statistical tests of a two-sided p-value of <0.05 were considered significant. Lastly, a geographical spatial analysis was performed to identify possible CHD diagnosis disparities across the state. Alabama ZIP code shape files were obtained from the Environmental Systems Research Institute living atlas (Redlands, CA) and all mapping and related geographical analysis was performed using ESRI’s ArcGIS Online and ArcGIS Pro programs. The benchmark we set for this study was the national average of antenatal diagnosis for the overall and certain CCHDs reported from STS data [11]. Prevalence of antenatal diagnosis at the ZIP code level was mapped throughout the state.
3. RESULTS
There were 1733 children with CHD who underwent a cardiac surgical repair. Of these, 20.2% (347) of the families knew of the child’s CHD diagnosis before birth and 79.8% (1386) did not. Baseline characteristics are described in Table 1. Those with an antenatal diagnosis were younger at the time of surgery than those who did not have a CHD diagnosis before birth (0.8±2.1 vs. 3.5±5.4 years; p <0.0001). Over 50% of those with an antenatal diagnosis had a STAT score of 3 or higher, while only 26% of those without an antenatal diagnosis had a 3 or higher STAT score (p <0.0001). Of those who did have an antenatal diagnosis, 23% had HLHS, while only 7.4% of those without a CHD diagnosis before birth had HLHS, Pulmonary Atresia (PA), and Tricuspid Atresia (TA). The distribution among those with no antenatal CHD diagnosis was ~5% TGA, ~11% TOF and ~65% all other CHDs (p <0.0001). There were no genetic, birth weight, race, preterm delivery status, health insurance, distance to clinic, and median ZIP code income or rurality status differences observed by antenatal diagnosis.
| - | Antenatal Diagnosis | - | |
|---|---|---|---|
| - | Yes | No | - |
| Variables | 347 (20.2) | 1386 (79.8) | p value |
| Age at first operation (years) | 0.8 ± 2.1 | 3.5 ± 5.4 | <0.0001 |
| Gender (male) | 183 (52.7) | 725 (52.3) | 0.95 |
| Race | - | - | 0.09 |
| White | 239 (69.7) | 869 (64.3) | - |
| Black | 99 (28.9) | 444 (32.8) | - |
| Other | 5 (1.4) | 39 (2.3) | - |
| Ethnicity | - | - | - |
| Hispanic | 19 (5.5) | 80 (5.8) | 0.81 |
| Birth weight (kg) | 2.9 ± 0.6 | 2.9 ± 0.8 | 0.17 |
| Pre-term birth | 56 (16.8) | 602 (47.5) | 0.14 |
| CHD diagnosis | - | - | <0.0001 |
| TGA | 26 (7.5) | 73 (5.3) | - |
| PA, TA, HLHS | 80 (23.0) | 102 (7.4) | - |
| TOF | 48 (11.6) | 161 (11.6) | - |
| All other CHDs | 184 (55.6) | 1026 (75.8) | - |
| STAT score | - | - | <0.0001 |
| 1 | 56 (18.5) | 602 (47.5) | - |
| 2 | 76 (25.1) | 324 (25.6) | - |
| 3 | 39 (12.9) | 122 (9.6) | - |
| 4 | 99 (32.7) | 200 (15.8) | - |
| 5 | 33 (10.9) | 19 (1.5) | - |
| Health insurance status† | - | - | 0.21 |
| Medicaid | 92 (51.1) | 392 (53.9) | - |
| Private | 76 (42.2) | 309 (42.5) | - |
| Veterans affairs | 5 (2.78) | 16 (2.2) | - |
| None | 7 (3.9) | 11 (1.5) | - |
| Have chromosomal abnormality | 119 (34.3) | 394 (28.4) | 0.32 |
| Median ZIP code income (USD) | $43,988 [36,448-56,707] | $42,861 [35,809-55,683] | 0.27 |
| Distance to clinic (miles) | 4.8 [2.2-13.6] | 4.8 [2.2-11.0] | 0.50 |
| Rurality | - | - | 0.27 |
| Rural | 52 (15.4) | 173 (13.1) | - |
| Urban | 286 (84.6) | 1146 (86.9) | - |
Key: TGA=Transposition of the great arteries, PA=Pulmonary atresia, TA=Tricuspid atresia, HLHS=Hypoplastic left heart syndrome, TOF=Tetralogy of Fallot.
| - | Odds Ratio† | 95% CI | Odds Ratio‡ | 95% CI |
|---|---|---|---|---|
| Race | - | - | - | - |
| Black vs. White | 1.3 | (0.96, 1.65) | 1.1 | (0.69, 1.66) |
| Other race vs. White | 2.8 | (0.98, 8.07) | 4.2 | (0.48, 37.91) |
| Health insurance | - | - | - | - |
| None vs. Private | 0.3 | (0.09, 1.14) | 0.2 | (0.05, 1.87) |
| Medicaid vs. Private | 1.2 | (0.86, 1.68) | 1.0 | (0.67, 1.61) |
| Veteran affairs vs. Private | 0.5 | (0.24, 1.44) | 0.3 | (0.10, 1.15) |
| Median ZIP code income | 1.0 | (1.00, 1.00) | 1.0 | (1.00, 1.00) |
| Distance to the clinic | 1.0 | (0.99, 1.07) | 0.9 | (0.97, 1.01) |
| Rural vs. Urban | 1.1 | (0.84, 1.46) | 1.0 | (0.63, 1.68) |
Regression analysis adjusted for age and the overall adjusted multivariable analysis yielded no significant associations between variables and not receiving an antenatal diagnosis (Table 2). The secondary analysis for differences in mortality among specific CCHD by antenatal diagnosis is shown in the supplemental table. There were 182 HLHS, TAs and/or PAs in total, of which only 43.9% had a known CHD diagnosis before birth; of the total 209 TOFs, only 22.9% had an antenatal diagnosis, and of the total 99 TGAs, only 26.2% had a known CHD diagnosis before birth. There were no significant differences in 30-day mortality among these CCHDs by antenatal status. Of those who died, the surgical mortality rates were consistently higher across all CCHDs among those who did not have an antenatal diagnosis when compared to those who did. Although there was no surgical or 30 days mortality among TOF and TGA children with a known diagnosis, p-values were unable to be calculated due to nonexistent or small numbers.
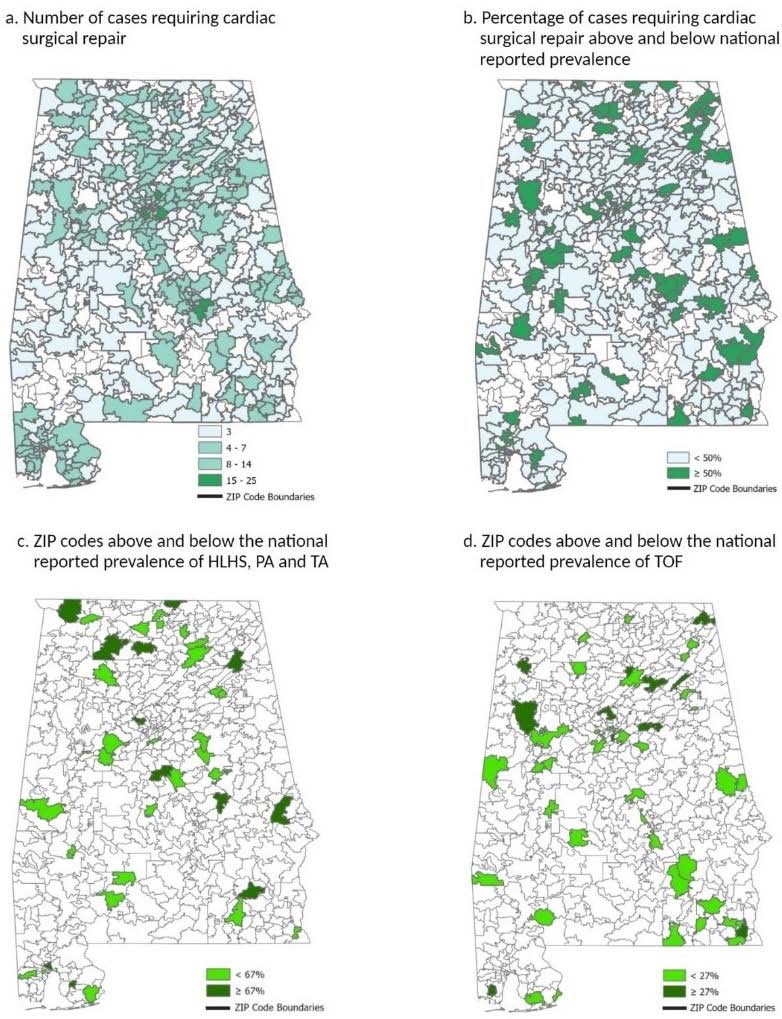
The geographical spatial analyses are shown in Fig. (2). There are 641 ZIP codes in the state of Alabama, of which 420 ZIP codes (65.5% of the state) reported at least 1 case of CHD. 83% (349) of these ZIP codes did not meet the national average of 50% CHD antenatal diagnosis benchmark (Fig. 2a and 2b) [9]. Of ZIP codes not meeting the 50% benchmark, 38.11% (133) were rural and 61.89% (216) were urban; for those that did meet the baseline, 46.48% (33) were rural and 53.52% (38) were urban (Pr>X2 = 0.189).
Figs. (2c and 2d) show the ZIP codes above and below the expected detection prevalence for that CHD. We found that 67.5% and 69.6% of ZIP codes in Alabama with reported cases did not meet the national antenatal diagnosis prevalence for HLHS and TOF, respectively. We were unable to construct a map for TGAs as no ZIP codes within the state were found to report more than one case within the time period of the study. The analysis also featured a side-by-side comparison with counties within the state based on predominant racial makeup and median income. While overlap was found, there was no statistically significant clustering.
4. DISCUSSION
In Alabama, only 20% of all children with CHD who required cardiac surgical repair were diagnosed antenatally. Although our analysis yielded no significant associations between the prevalence of antenatal diagnosis and a broad array of social, demographic, surgical outcomes and geographical factors, including insurance, income, and race, 83% of the state’s ZIP codes underperformed at meeting the national average rate of antenatal CHD diagnosis. About 68% of the counties underperform in detecting specific critical CHD that may require immediate surgery after birth. Only 43% of children with single ventricle physiology (HLHS, PA, TA) had a diagnosis before birth compared to the national 67% rate. Despite initiatives implemented to ensure prenatal health care regardless of insurance status, CHDs still remain largely undiagnosed prenatally in our state.
It is estimated that 8% of live births in Alabama occur to women with late or no prenatal care, suggesting that these women are not receiving routine ultrasounds in an optimal setting and/or time window that could diagnose their fetus with a CHD, as displayed in Fig. (1). In Alabama, around 21.5% of births have been to women beginning prenatal care in the second trimester and 7.9% have been to women receiving late or no prenatal care. Prenatal care visits are a pre-requisite to getting a prenatal ultrasound. Of live births in Alabama in 2019, 19.0% were born to women classified as receiving inadequate prenatal care [24]. Eighteen counties in the state in 2013 had a proportion of births with inadequate prenatal care at or higher than 30%, while a high prevalence of poor pregnancy outcomes was outlined as the third largest health concern for the state [25]. Another possible factor that may be influencing the low CHD diagnosis prevalence antenatally is the lack of uniform training across the state for those performing fetal ultrasounds. Generally, ultrasound technicians are licensed in addition to receiving formal education, with additional training provided specifically for those who want to assist in or perform fetal echocardiograms. The state of Alabama has no specific specialty certification requirements for ultrasound technicians, though some specific facilities do [26]. Coupled with the lack of training for ultrasound technicians in some areas of the state, there is relatively low obstetrician/gynecologist (OB/GYN) coverage across the state of Alabama. Additionally, there are differences in who reads fetal ultrasounds among healthcare providers. For example, radiologists read fetal ultrasounds in some areas and have lower fetal ultrasound volume than areas where OB/GYNs or Maternal-Fetal Medicine specialists read fetal sonograms. While this shortage is much more pronounced in rural areas, the geography of Alabama makes this a state-wide issue. Because the majority of the state of Alabama is considered a medically underserved area, the permeation of the issue across all indicators can be partially explained [27]. A third factor to consider for low antenatal detection rates is obesity, as Alabama has one of the highest rates of obesity in the nation [28]. Obesity in pregnant women is associated with an increased risk for birth defects, including CHD. Visualization, which is necessary for the detection of CHD, is further decreased by the absorption of ultrasound waves into the proximal abdominal adipose tissue [29-31]. We speculate that access to care issues, systems factors, and individual influences contribute to the high rate of postnatally diagnosed CCHDs [25].
Geographical variations in the efficacy of prenatal screening for CHD have been described in the United States [11]. While the results from the cited study only show patients who underwent surgery for severe CHD and are shown on a state-by-state basis, it does reveal a disparity among states, with examples including Pennsylvania showing an efficacy of >50%, Nebraska showing an efficacy of <20%, and Alabama showing an efficacy between 30% and 40% in 2017 [11]. One study of an urban area in the U.S. estimated that a pregnant woman of high risk and using public transportation could cumulatively spend over $100 (USD) on travel covering 243.6 miles over the course of 25.5 hours [32]. A study conducted in the state of Georgia, located in the Southern U.S., showed discrepancies in access to prenatal care via spatial accessibility scores between rural and urban census-tracts [33]. Lastly, a study highlighting access to prenatal care in New York City utilized Kernel estimates of the density of prenatal clinics to census tracts of pregnant women and mothers on Medicaid. This study indicated that having a higher density of clinics nearby was associated with a lower risk of either starting prenatal care late or not starting at all [34].
There have been no studies to the knowledge of the authors that have analyzed associated demographic factors of prenatal diagnosis at the scale of every reporting ZIP code in a single U.S. state. This research highlights the need for future studies that analyze a myriad of factors (ultrasound technician training, review of the optimal physician to review obstetric ultrasounds, socioeconomic status, prenatal care, and distance to the clinic) that may lead to a missed CHD diagnosis so that mothers and infants can receive the best care and preparation needed. Published studies have shown that a significant factor in successful prenatal diagnosis of CHD is both the experience level of the ultrasound operator and technological resources [4, 11, 15, 16]. In our own center, we previously evaluated the added value of a fetal echocardiogram after obtaining normal cardiac views on a fetal anatomic survey. In the hands of American Registry for Diagnostic Medical Sonography certified sonographers in an American Institute of Ultrasound in Medicine accredited ultrasound unit, 21 total CHDs in total were missed between January 2010 and June 2018. This amounted to 21 (1.7%) of 1,223 fetal echocardiograms. Importantly, three CCHDs (0.25%) were missed. Key points from this review include the following: prenatal detection of abnormal cardiac anatomy by trained sonographers is exceedingly high, and prenatal detection of CCHD in the hands of trained sonographers and physicians is high, though not perfect [35]. The question from a public health perspective remains as to how we can facilitate timely prenatal care and referral for high-risk patients. If a patient never receives prenatal care, there are no opportunities for prenatal diagnosis. If a patient accesses prenatal care late, resulting in a third-trimester anatomic survey, image acquisition may be hampered by gestational age and maternal habitus [36, 37].
Several studies have found associations between the uptake of antenatal care and both insurance status and distance to clinics; however, the current study found that these two factors may not imply a lower likelihood of getting a proper antenatal diagnosis [32-34, 38, 39]. Previous studies were performed in mostly urban areas where the disparity may be more pronounced between groups without private insurance or those living further away from clinics. Because the state of Alabama is largely rural, healthcare facilities are mostly remote, which may result in more heterogeneous patient characteristics.
The generalizability of this study may be limited as it only pertains to one state. Also, some CHDs are very rare, resulting in small numbers. In addition, our study did not account for CHDs among pregnancy terminations, fetal demise, postnatal deaths before surgery, or cases treated by catheter techniques. Another limitation of the data analysis comes from the small scale of geographic areas utilized. With ZIP codes generally holding a smaller population than counties, many have only reported one case within the 2013-2019 timeframe of our database while many others reported none. This made it difficult to create any clustering analysis that would not be biased. There may also be factors associated with the antenatal diagnosis that were unmeasured.
CONCLUSION
The prevalence of antenatal diagnosis of major CHD in Alabama is lower than the reported national average across all demographics. This analysis can serve as an example for other states to identify key geographical areas and possible factors for missed CHD diagnosis. Mitigation of factors relating to low antenatal diagnosis prevalence and the eventual improvement of these numbers can help prepare patients and parents, allow for delivery at an appropriate center, and improve neonatal outcomes. Future studies are needed to provide solutions to address the disparity between prenatal ultrasound rates and prenatal CHD diagnosis.
LIST OF ABBREVIATIONS
| CHD | = Congenital Heart Disease |
| CCHDs | = Structurally critical CHDs |
| STS | = Society of Thoracic Surgeons |
| TOF | = Tetralogy of Fallot |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Institutional Review Board with approval number IRB-110626007.
HUMAN AND ANIMAL RIGHTS
No animals were used for studies that are the basis of this research. All the human procedures used were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013 (http://ethics.iit.edu/ecodes/node/3931).
CONSENT FOR PUBLICATION
Due to the methodology of this study, the ethics board deemed this an exempt protocol and waived the need for consent.
STANDARDS OF REPORTING
STROBE guidelines were followed.
AVAILABILITY OF DATA AND MATERIALS
The data used for this research will not be made available for sharing. It is not publicly available and belongs to this institution.
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
FUNDING
None.
ACKNOWLEDGEMENTS
Declared none.


