All published articles of this journal are available on ScienceDirect.
Seroprevalence of SARS-Cov-2 Virus Infection In Kermanshah, Iran: A Population-based Cross-Sectional Study
Abstract
Background:
The aim of this study was to estimate the seroprevalence of SARS-CoV-2 infection in a general population from Kermanshah province, Iran.
Methods:
The present study was a population-based cross-sectional design conducted in Kermanshah province in 2020. Sampling was performed in a multi-stage process, and 1967 participants were considered, and also 174 interviewers were assigned to collect data online. Then, 5 mL of blood sample was taken from every participant. The blood samples were centrifuged with the ELISA method to detect SARS-CoV-2-specific IgG and IgM antibodies in serum samples. Seropositive prevalence was adjusted by means of survey analysis. Case fatality rate (CFR) and infection fatality rate (IFR) were estimated.
Results:
A total of 1967 people from 14 cities of the province participated in the study. The mean age of participants was 35.7±16.9, and 50.4% were female. The lowest and highest seroprevalence was found in the cities of Paveh (2.3% [0.3- 4.2]) and Harsin (61.6% [54.7–68.5]), respectively. The CFR and IFR in men and women were 3.4 vs. 3.3 and 0.1 vs. 0.3. The aged 60 years or older had the highest CFR and IFR with 11.2 and 3.7%, respectively.
Conclusion:
The prevalence of SARS-CoV-2 infection and IFR among the general population of Kermanshah province was 18.3 and 0.3%, respectively. The results of this study can assist the policymaker in assessing risk factors, and transmission dynamics of SARS-CoV-2 in a population and implementing preventive and control interventions.
1. INTRODUCTION
The 2019 coronavirus disease (COVID-19) pandemic is caused by a novel coronavirus named “Severe Acute Respiratory Syndrome Coronavirus 2” (SARS-CoV-2) and has become a major worldwide challenge since December 2019 [1]. The person-to-person transmission of COVID-19 occurs during the pre-symptomatic phase through direct contact or droplets [2-4]. Because asymptomatic cases can spread the infection in the population [5], the probability of transmission in the pre-symptomatic phase of this infection has led to challenges in controlled measures [6, 7]. Although Reverse Transcription of the Polymerase Chain Reaction (RT-PCR) is currently considered the “standard test” for diagnosing SARS-CoV-2 infection [8], studies have shown a significant number of asymptomatic or subclinical individuals remain undetected by RT-PCR. Thus, it is likely that the actual number of people exposed or infected are underestimated [9-11]. Due to the high proportion of asymptomatic and mild infections, it is difficult to estimate the true prevalence of the infection and its mortality rate [12].
Serological tests are important diagnostic methods, especially in patients with mild to moderate infected cases. The use of serological antibody tests, including immunoglobulin G (IgG) and M (IgM) can help estimate the true number of COVID-19 infections [13, 14]. The detection of SARS-CoV-2 specific IgG and IgM in blood serves as a method for determining whether the individual has been infected recently (IgM) or earlier (IgG); IgM usually increases after seven days of infection before increasing IgG. IgG is a longer-lasting antibody which develops 15 to 30 days after the onset of infection [11, 15]. Notably, several studies have reported that approximately 38 to 48% of the population with seropositivity had no symptoms of Covid-19 [16-18]. Therefore, the identification of IgM and IgG antibodies against SARS-CoV-2 can be considered a complementary method for diagnosis and play a significant role in assessing immune responses against SARS-CoV-2 infection. Furthermore, due to the persistence of antibodies to SARS-CoV-2 (mainly IgG) after virus clearance, a serological method provides accurate data to estimate the prevalence of SARS-CoV-2 infection and exposure to it in a population. Given that the seroprevalence of infection reflects the immune status of individuals or the community [19], it can assist policymakers in designing and implementing prevention interventions, quarantine, and predicting medical facilities. Therefore, the aim of this study was to evaluate the seroprevalence of SARS-CoV-2 infection in the general population of Kermanshah province in 2021.
2. MATERIALS AND METHODS
2.1. Study Design and Study Setting
The present study was a population-based cross-sectional design to estimate the seroprevalence of antibodies against SARS-CoV-2 in Kermanshah province from November 15th to December 20th, 2020. Kermanshah province is one of the 31 provinces of Iran located in the west of Iran and composed of fourteen districts. According to the 2016 census, the general population of Kermanshah is about 2 million people, and the inhabitants are scattered over 24,998 km2.
2.1.1. Sample Size and Sampling Strategy
The sample size of this study was calculated as follows the formula:
n=z2 (p (1-P)/d2) = 1990
Z: for 95% confidence level is 1.96
P: Prevalence of positive COVID-19 based on the results of previous seroepidemiological studies in Kermanshah (15), which was 15%.
d: d is the absolute precision, also called the margin of error, which was taken into account in a study of one-quarter of the prevalence, which was 0.0157.
Finally, considering the high population of Kermanshah city as the capital of the province, 700 people were assigned to it, and 100 people were considered for each of the other 13 cities.
The first classification stage is based on geographical location and determining regions. The second stage of the quota sample: according to the population of each region, the city (town and village) of each section was determined. The third stage of systematic sampling: Based on the cumulative list (population) of health centers and houses in each city, the centers in which the sample was placed were determined in a systematic method. Fourth step: In the health centers, based on the list of households, the head of the cluster was randomly determined, and according to the place of residence of the head of the cluster, 9 neighboring households were also added. After selecting the head of the cluster, a phone call was made to the family through the health care/health care provider, and based on the family's desire, the questionnaire was completed by inviting them to the center or visiting them at home. In case of the unwillingness of the household, it was replaced from the same place of the household. The required number of samples was divided into clusters of 10 people, 2000 samples included 200 clusters, and to select the head of each cluster, 10 samples (5 women and 5 men) were selected from the age groups of 15-29, 30-49 and over 50 years. Unfortunately, 25% of the selected individuals were reluctant to participate and were consequently replaced. In total, data were collected from 1967 participants. All study participants provided written informed consent; for participants <18, consent was obtained from their parents or guardians.
2.2. Data Collection and Research Instrument
Demographic and epidemiological data were collected by employing World Health Organization (WHO) approved questionnaire and translation into the Persian language. This questionnaire has already been used elsewhere [16]. This questionnaire was designed to include questions on demographics (personal information, age, sex, city, rural/urban, job, education, height, weight, blood group, family size, role in the family), history of infection (self-reported history of infection, Physician's diagnosis of COVID-19 infection, diagnosis method, admission history, close contact, smoking, alcohol consumption, opium use,…), symptoms (cough, sputum cough, hemoptysis, fever, chills, sore throat, headache, distress, diarrhea, loss of smell, loss of taste, nausea, vomiting, weakness, muscular pain, convulsions, chest pain, runny nose, consciousness, anorexia, dermatitis, plegia, and others), underlying diseases (CVDs, hypertension, COPD, asthma, obesity, fatty liver, cirrhosis, malnutrition, IBD, hepatitis B, hepatitis C, autoimmune hepatitis, HIV/AIDS, renal failure, dialysis, thalassemia, hemophilia, dementia, MS, cancer, organ transplants, and other), and protocol compliance (face mask use, skin disinfectant use, hand washing, receiving hospital services, visiting clinic/emergency room, school/university, living in elderly care center, use of vehicles, participation in communities, maintaining physical distance,…). People were invited by telephone to participate in the study, and if they agreed to participate in the study, they were questioned by the interviewers (174 people) with a tablet and their information was recorded online. Collected data were linked to the covid registry system in order to obtain a history of infection and date of infection. A history of infection means having a positive RT-PCR or CT scan with symptoms of COVID-19.
2.3. Inclusion and Exclusion Criteria
All individuals were brought into the study regardless of previous COVID-19 infection history. The exclusion criterion consisted of the unwillingness to participate in the study, inability to answer the questions, and residence in that area for less than a year.
2.4. Lab Data
After the interview, 5 mL of blood sample was taken from every participant. The blood samples were centrifuged, and the serums were separated and immediately stored at -80 °C. According to the manufacturer's instructions, detecting SARS-CoV-2-specific IgG and IgM antibodies in serum samples was performed by using enzyme immunoassay (ELISA) and assay (PISHTAZTEB, Tehran, Iran) [20].
All blood samples were tested in the central laboratory of Kermanshah University of Medical Sciences. The manufacturer-reported sensitivity of the SARS-CoV-2 IgM ELISA capture kit was 30.7%, 85.4%, and 78.4%, respectively, in samples taken 0-6, 7-14, and more than 15 days from symptom onset and a specificity of 99.4% [20]. The sensitivity and specificity of the ELISA kits were, respectively, 94.1% and 98.3% for the SARS-CoV-2 IgG indirect ELISA kit. The seroprevalence of COVID-19 was adjusted for test performance. Results were interpreted as follows: cutoff index <0.9, negative and cutoff index ≥1.1, positive for anti-SARS-CoV-2 antibodies. The definition of seroprevalence in our project is IgG or IgM positive. IgM indicates a recent infection and IgG indicates a history of infection. Also, the infection fatality ratio (IFR) and case fatality ratio (CFR) defines the risk of death per infection and per case, respectively. Trained professionals did all the steps considering appropriate distancing, and all participants used the recommended personal protective equipment.
2.5. Statistical Analysis
Variables were presented as numbers (percentages) between the subgroups of demographic and seropositivity prevalence. Seropositivity prevalence was indicated by percent and a 95% confidence interval. Since participants were recruited from different localities, the cities were considered sampling units, and their clustering effects were adjusted using survey analysis.
Morbidity and death were extracted from the covid registry system according to sex, age, and city in Kermanshah provinces. Also, the infection in the population was estimated to consider the seropositivity and case fatality rate and infection fatality rate as calculated. The Stata software version 14 was used for statistical analysis. Plus, In the course of the analysis, implausible range data and outliers were taken into consideration in Excel version 2019. Outliers are data points that are far from other data points and implausible ranges are unusual values in a dataset.
3. RESULTS
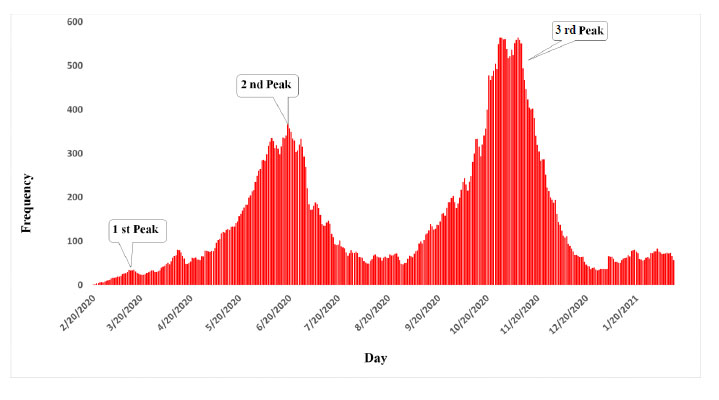
Kermanshah province has experienced three peaks of the COVID-19 epidemic so far, and according to prediction, the fourth peak is also possible (Fig. 1). A total of 1967 people from 14 cities of the province participated in the study during the third peak. 25% of the selected individuals were reluctant to participate and were consequently replaced. The mean age of participants was 35.7±16.9, and 50.4% of participants were female.
Table 1 shows the seroprevalence of SARS-CoV-2-specific antibodies according to the demographic characteristics of the population under study. The seroprevalence of IgG or IgM antibodies in men and women was 184 (18.6) and 214 (21.9), respectively. The highest seroprevalence of IgG or IgM was observed in individuals aged 60 years or older (22.3%) but not much different from other age groups. One hundred twenty-six participants had a history of Covid 19, 50% of whom had IgG or IgM antibodies. Also, 11.9% of them had high IgM antibodies, which is a sign of a recent infection. The seroprevalence of IgG or IgM in the urban population was higher than rural population (22.8 vs. 15.3%) and this difference is statistically significant (p=<0.001). Also, serum prevalence based on a history of close contact, previous infection, and symptoms were 20.8, 4.0, and 29.2 percent, respectively. These differences are statistically significant (p=<0.001)
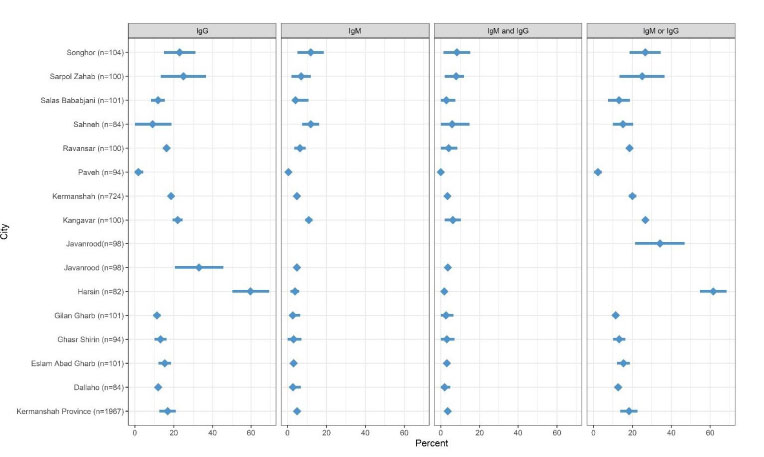
Fig. (2) shows the sample size and seroprevalence of SARS-CoV-2-specific IgG and IgM antibodies. As can be seen, the lowest IgM, IgG, IgM and IgG, and IgM or IgG seroprevalence was found in Paveh, 0.5, 1.8, 0, and 2.3, respectively. On the other hand, the highest IgM seroprevalence was found in Sahneh (11.9% [7.5–16.3]) and Songhor (5% [5–18.7]) cities. Also, the highest IgG seroprevalence was found in Harsin (59.7% [50.1–69.4]). In addition, the highest seroprevalence of SARS-CoV-2-specific IgG and IgM and IgM or IgG antibodies were related to Songhor (8.3% [1.4–15.2]) and Harsin (61.6% [54.7–68.5]); respectively.
Table 2 demonstrates the Case Fatality Rate (CFR) and Infection Fatality Rate (IFR) in the population under study based on sex, age, and city. As illustrated, the CFR and IFR in men and women were 3.4 vs. 3.3 and 0.1 vs. 0.3, respectively. The highest CFR and IFR were observed in individuals aged 60 years or older, with 11.2 and 3.7%, respectively. In addition, the highest and lowest rates of CFR were found in Dallaho and Salas Bababjani cities, with 6 and 2.1%, respectively. Likewise, the highest and lowest rates of IFR were found in Gilan Gharb and Salas Bababjani cities, with 0.8 and 0%, respectively.
| Variable | Categories | No. of Subjects (%) | Only IgM(95% CI) | Only IgG(95% CI) | IgM and IgG (Simultaneous) (95% CI) | IgM or IgG (95% CI) | P-value for IgM or IgG |
|---|---|---|---|---|---|---|---|
| Sex | Female | 991 (50.4) | 32 (3.2) | 173 (17.5) | 21 (2.1) | 184 (18.6) | 0.06 |
| Male | 976 (49.6) | 57 (5.8) | 193 (19.8) | 36 (3.7) | 214 (21.9) | ||
| Age | <15 | 242 (12.3) | 11 (4.5) | 47 (19.4) | 6 (2.4) | 52 (21.5) | 0.75 |
| 15-45 | 1135 (57.7) | 43 (3.8) | 203 (17.9) | 25 (2.2) | 221 (19.5) | ||
| 45-60 | 397 (20.2) | 18 (4.5) | 77 919.4) | 13 (3.3) | 82 (20.6) | ||
| >=60 | 193 (9.8) | 17 (8.8) | 39 (20.21) | 13 (6.7) | 43 (22.3) | ||
| Education | Illiterate | 231 (11.7) | 13 (5.6) | 42 (18.2) | 8 (3.5) | 47 (20.35) | 0.99 |
| Less than a diploma | 916 (46.6) | 45 (4.9) | 165 (18.0) | 28 (3.0) | 182 (19.9) | ||
| Diploma | 539 (27.4) | 20 (3.7) | 103 (19.1) | 12 (2.2) | 111 (20.6) | ||
| Bs | 230 (11.7) | 6 (2.6) | 44 (19.1) | 4 (1.7) | 46 (20.0) | ||
| MSc and over | 51 (2.6) | 5 (9.8) | 12 (23.5) | 5 (9.8) | 12 (23.5) | ||
| Job | Homemaker | 684 (34.8) | 24 (3.5) | 117 (17.1) | 16 (2.3) | 125 (18.2) | |
| Providing services | 596 (30.2) | 35 (5.8) | 128 (21.5) | 34 (5.7) | 139 (23.3) | 0.41 | |
| Military staff | 13 (0.7) | 0 (0.0) | 4 (30.8) | 0 (0.0) | 4 (30.8) | ||
| Healthcare staff | 17 (0.9) | 2 (11.7) | 3 (17.6) | 0 (0.0) | 5 (29.4) | ||
| Other | 657 (33.4) | 28(4.3) | 114 (17.3) | 62 (9.4) | 125 (19.0) | ||
| Previous infection | Yes | 126 (6.4) | 15 (16.8) | 61 (16.6) | 13 (22.8) | 63 (4.0) | <0.001 |
| No | 1841 (93.6) | 115 (5.9) | 65 (4.0) | 113 (5.9) | 63 (15.8) | ||
| Residence type | Urban | 1287 (65.4) | 59 (4.6) | 279 (21.7) | 44 (3.4) | 294 (22.8) | |
| Rural | 680 (34.6) | 30 (4.4) | 87 (12.8) | 13 (1.9) | 104 (15.3) | <0.001 | |
| Close contact | Yes | 200 (10.2) | 18 (9.0) | 80 (21.8) | 15 (26.3) | 83 (20.8) | <0.001 |
| No | 1767 (89.8) | 182 (9.7) | 120 (7.5) | 185 (9.7) | 117 (7.5) | ||
| Family size | <=2 | 250 (12.7) | 10 (4.0) | 47 (18.8) | 7 (2.8) | 50 (20.0) | |
| 7-Feb | 1624 (82.6) | 75 (4.62) | 298 (18.35) | 48 (3.0) | 325 (20.0) | 0.54 | |
| >=7 | 93 (4.7) | 4 (4.3) | 21 (22.6) | 2 (2.1) | 23 (24.7) | ||
| Smoking | Yes | 104 (5.3) | 4 (4.5) | 15 (4.1) | 1 (0.2) | 18 (4.5) | 0.44 |
| No | 1863 (94.7) | 100 (5.3) | 89 (5.5) | 103 (5.4) | 86 (5.5) | ||
| Alcohol consumption | Yes | 11 (0.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.09 |
| No | 1956 (99.4) | 11 (0.6) | 11 (0.7) | 11 (0.6) | 11 (0.7) | ||
| Opium Use | Yes | 42 (2.1) | 2 (4.7) | 6 (14.3) | 0 (0.0) | 8 (19.0) | 0.84 |
| No | 1925 (97.9) | 40 (2.1) | 36 (2.2) | 42 (2.2) | 34 (2.2) | ||
| Having symptoms | Yes | 363 (18.45) | 16 (4.4) | 102 (28.1) | 12 (3.3) | 106 (29.2) | <0.001 |
| No | 1604 (81.5) | 347 (18.5) | 261 (16.3) | 351 (18.4) | 257 (16.4) | ||
| Comorbidity | Yes | 342 (17.4) | 15 (16.8) | 65 (17.8) | 11 (19.3) | 69 (17.3) | 0.97 |
| No | 1625 (82.6) | 327 (17.4) | 277 (17.3) | 331 (17.3) | 273 (17.4) |
| - | Categories | Morbidity * | Death * | Estimation of Infection in the Population$ | Case Fatality Rate | Infection Fatality Rate |
|---|---|---|---|---|---|---|
| Sex | Female | 17970 (42.9) | 587 (41.6) | 179382 (45.4) | 3.3 | 0.3 |
| Male | 23949 (57.1) | 823 (58.4) | 988015 (54.6) | 3.4 | 0.1 | |
| Age | <15 | 835 (2.0) | 19 (1.4) | 91526 (23.2) | 2.3 | 0 |
| 15-45 | 22492 (53.6) | 113 (8.0) | 195000(49.5) | 0.5 | 0.1 | |
| 45-60 | 9091 (21.7) | 217 (15.4) | 78828 (20.0) | 2.4 | 0.3 | |
| >=60 | 9501 (22.7) | 1061 (75.2) | 28498 (7.3) | 11.2 | 3.7 | |
| Kermanshah Province | 41919 | 1410 | 357295 | 3.4 | 0.3 | |
| Eslam Abad GHarb | 2820 (6.7) | 156 (11.1) | 21695 (5.0) | 5.5 | 0.7 | |
| Paveh | 1359(3.2) | 61 (4.3)) | 13899 (3.2) | 4.5 | 0.4 | |
| Salas Bababjani | 439 (1.0) | 9 (0.6) | 46141(1.0) | 2.1 | 0 | |
| Kermanshah | 25550 (61.0) | 704 (49.9) | 220000 (51.0) | 2.8 | 0.3 | |
| Javanrood | 1476 (3.5) | 36 (2.6) | 25807 (5.9) | 2.4 | 0.1 | |
| Dallaho | 419 (1.0) | 25 (1.8) | 4570 (1.0) | 6 | 0.5 | |
| Sarpol Zahab | 1671 (4.0) | 56 (4.0) | 21335 (4.9) | 3.4 | 0.3 | |
| Sahneh | 1107 (2.6) | 45 (3.2) | 10755 (2.5) | 4.1 | 0.4 | |
| Ravansar | 752 (1.8) | 39 (2.8) | 8817 (2.0) | 5.2 | 0.4 | |
| Gilan Gharb | 1052 (2.5) | 55 (3.9) | 6499 (1.5) | 5.2 | 0.8 | |
| Kangavar | 1446 (3.4) | 75 (5.3) | 20350 (4.7) | 5.2 | 0.4 | |
| Ghasr Shirin | 435 (1.0) | 17 (1.2) | 3158 (0.07) | 3.9 | 0.5 | |
| Songhor | 1926 (4.6) | 56 (4.0) | 21722 (5.0) | 2.9 | 0.3 | |
| Harsin | 1467 (3.5) | 76 (5.4) | 42264 (11.2) | 5.2 | 0.2 |
The results of this study also showed the self-report for morbidity, history of close contact, close contact with a family member with a seropositive test and a history of the previous infection was 126 (6.4), 200 (10.2), 182 (9.25%), respectively. In addition, 150 (41.3%) and 81 (23.7%) of these subjects had at least one symptom of coronavirus and at least one underlying disease, respectively. In people with a seropositive test or history of the previous infection, 126 (6.4%) were diagnosed by a physician, and 6 (0.3%) had a history of hospital admission.
4. DISCUSSION
This study aimed to determine the SARS-CoV-2 antibody seroprevalence in western Iran's general population of Kermanshah province. IgM and IgG antibodies are often developed simultaneously against SARS-CoV-2 in the early stage of the disease; however, those could be detected only in 12.5% of patients on the first day of hospitalization [20]. IgG and IgM levels peak thirty days after the onset of symptoms, then IgM begins to decline, and by the third month, 30% of patients' tests are negative. IgG levels will be high for up to 3 months and drop dramatically in the seventh month [20, 21]. On the other hand, seroconversion depends on several factors, including age, gender, the severity of the disease, and the underlying diseases [22]. This finding suggests that simultaneous detection of IgM and IgG antibodies against SARS-CoV-2 could be a way to improve the diagnosis of infection.
This study is the first population-based serological survey for both active and past SARS-CoV-2 infections in Kermanshah province and has estimated the seroprevalence of IgM or IgG of 18.3 (95% CI: 13.7- 22.7) by late December 2020 (during the third peak). In the present study, a geographical variation was observed in estimating the seroprevalence, which ranged from 2.3 to 61.6%. Our seroprevalence estimate was less than that reported from Semnan (19.3%, 95% CI, 14.0-26.7) in late June 2020 [14] and more than Mazandaran's 15.26% (95% CI: 12.97%-17.79%) in May 2020 [15]. Between April 17th and June 2nd, 2020, in a large and population-based seroprevalence study using obtained sera samples from 5372 individuals with a high risk of exposure to SARS-CoV-2 and 3530 individuals from the general population in 18 cities in 17 Iranian provinces, adjusted seroprevalence was estimated 20% (18.5–21.7) and 17.1% (95% CI 14.6–19.5) ranged from 72.6% (95% CI: 53.9–92.8) to 1.7% (95% CI: 0·0–6·0), respectively [16]. In the above study, the seroepidemiology of the SARS-CoV-2 in Kermanshah city, the capital of the province, in the general population and high-risk groups were 17.3% (95% CI: 5.3-30.9) and 11.9 (95% CI: 7.6-16.4); respectively [16].
A large percentage of Kermanshah residents adhere to the protocols so during the study period, 83% of participants always used masks, 12% occasionally, and only 5% did not use masks. The corresponding values for adherence to physical distancing were 68%, 19%, and 13%. Also, 90% of the participants reported not participating in any gatherings. However, according to the results of this study, almost one-fifth of the residents of the Kermanshah province have experienced COVID-19, which is not enough to achieve herd immunity.
To estimate COVID-19 transmission from asymptomatic individuals, an estimation of the asymptomatic rate seems necessary. We found that almost one-sixth of seropositive individuals did not report COVID-19-related symptoms, and almost 80% did not report contact with an individual with a confirmed COVID-19 infection. The rate of asymptomatic infections ranged from 6.3% to 96.0% in a systematic review [21]. Therefore, seroprevalence studies may be helpful tools for understanding the prevalence of COVID-19 infection in populations where the large majority of individuals are asymptomatic.
We found that the seroprevalence of IgM or IgG among males was 21.9%, which was numerically higher than females with 18.6%. Other studies in Italy [17] and the United Arab Emirates [22] showed that seroprevalence was higher in males than in females. In contrast, a population-based nationwide survey in Iran showed weighted seroprevalence is higher in females than in males (13.2%, 11.1–15.3 vs. 12.5%, 10.5–14.7) [16]. A systematic review and meta-analysis conceded that the seroprevalence does not differ significantly between males and females [18]. However, demographic and social factors may profoundly influence mobility across cultures. Hence higher incidences of SARS-CoV-2 infection may be related to mobility function rather than age and gender. We observed a similar prevalence between individuals in the age group 0-19 and the older age group, which was consistent with the previous reports [19].
Some studies have reported an increased risk of COVID-19 infection among working-aged populations [23, 24]. Though the risk of severe illness or death with COVID-19 increases with age [25], we found the risk of death increase with age. The authors also failed to find evidence that the infection rates differ by education, household size, or comorbidity; However, the infection rates were higher among healthcare workers [26, 27], which was inconsistent with other studies in Iran and the world.
We estimated IFR = 0.3%, which was higher than the rates estimated for South India (0.13%) [24] and the Maranhão in Italy (14%) [28]. Estimating IFR is a challenge as it will depend on the sensitivity and specificity of the test used, age structures, different processes of testing and reporting death, and selective testing of high-risk populations. Therefore, IFR is considerably different across populations.
It is noteworthy that at the time of performing the study, vaccination against COVID-19 had not started in the country, and the first vaccination began on February 20th, 2021, with the priority on medical staff, the elderly population, and high-risk groups. Due to the deficiency of vaccines in Iran, vaccination is slow, and it is challenging to achieve herd immunity through vaccination. However, based on our results, it is estimated that one-fifth of the province's population has been exposed to the SARS-CoV-2 infection, and this number has undoubtedly slightly increased since the survey was completed last December 2020. Although IgG and neutralizing antibodies induced by SARS-CoV-2 infection are protective, several studies have reported subsequent SARS-CoV-2 infection [29, 30]. Nevertheless, Letizia et al. observed that the risk of COVID-19 is five times higher for seronegative young adults than for those with seropositivity [31]. However, the duration of immunity against COVID-19 in seropositive individuals remains uncertain, and protection may decline over time.
This study has several limitations. As mentioned earlier, 25% of those invited were reluctant to participate in the study and were replaced. In addition, data regarding risk factors such as comorbidity, symptom-related COVID-19, history of contact with COVID-19-confirmed individuals and illnesses were based on self-reporting. On the other hand, this study was performed on a large sample size (1967 participants) with high generalizability. Also, participants in all age groups and proportion to size were investigated, which can be the strength of this research.
CONCLUSION
In conclusion, the prevalence of SARS-CoV-2 infection and IFR in the general population of Kermanshah Province at the end of the third wave of SARS-CoV-2 were 18.3 (13.7- 22.7) and 0.3%; respectively. The results of this study can assist the policymaker in assessing risk factors, transmission dynamics of SARS-CoV-2 in a population, and implementing preventive and control programs.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Deputy of the Research and Ethics Committee of Kermanshah University of Medical Sciences (Iran) (ID: IR.KUMS.REC.1399.565).
HUMAN AND ANIMAL RIGHTS
No animals were used for studies that are the basis of this research. All the humans were used in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), with the Helsinki Declaration of 1975, as revised in 2013 (http://ethics.iit.edu/ecodes/node/3931).
CONSENT FOR PUBLICATION
All study participants provided written informed consent; for participants <18, consent was obtained from their parents or guardians.
STANDARDS OF REPORTING
STROBE guidelines were followed.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article is available in the Kermanshah Health Observatory at http://digit.kums.ac.ir/q/3010424.html. The corresponding author has access to the data.
FUNDING
The study was supported by the Deputy of Research of Kermanshah University of Medical Sciences, Kermanshah, Iran.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
| COVID-19 | = Coronavirus Disease |
| SARS-CoV-2 | = Severe Acute Respiratory Syndrome Coronavirus 2 |
| RT-PCR | = Reverse Transcription of the Polymerase Chain Reaction |


