All published articles of this journal are available on ScienceDirect.
The Difference in Mouthwash Side Effects of Persica and Chlorhexidine for Preventing Ventilator-induced Pneumonia among Patients Admitted to the Intensive Care Unit
Abstract
Background:
Pneumonia is a common cause of morbidity and mortality in ICU patients under mechanical ventilation. In recent years the use of herbal mouthwashes, due to antimicrobial effects and fewer side effects, has been studied in reducing the incidence of ventilator-associated pneumonia (VAP). In this study, the effect of Persica mouthwash in the prevention of VAP in ICU patients was compared with chlorhexidine.
Methods:
This study is a double-blind, randomized clinical trial among ICU patients under mechanical ventilation. Fifty patients were divided into two groups, the control group used 10 ml Chlorhexidine 2.0%, and the intervention group used 10cc Persica as a mouthwash. The incidence of pneumonia, mortality, length of hospital stays, mechanical ventilation duration, CPIS (Clinical Pulmonary Infection Score), and SOFA (sequential organ failure assessment) score and complications were evaluated among the two groups.
Results:
The incidence of pneumonia, mortality, SOFA score, and CPIS in the two groups were not significantly different. Length of stay in the ICU and mechanical ventilation duration were also not significantly different in the two groups, p>0.05. Side effects with chlorhexidine were significantly more often than Persica (44% vs. 8%) p=0.008.
Conclusion:
The incidence of early pneumonia in patients with no baseline pneumonia did not differ with Persica and Chlorhexidine mouthwash. At the same time, the incidence of side effects caused by the use of Persica was significantly less.
Clinical Trial Registrations No.:
RCT2017022032676N1.
1. INTRODUCTION
Ventilation-associated pneumonia is one of the common complications among intensive care unit (ICU) patients who are under mechanical ventilation. It is associated with a greater number of morbidities and reduces survival in these patients [1]. 28% of patients under ventilation develop VAP within 48 hours, and 24-50% of patients die because of VAP [2].
Oral colonization of pathogens in 63% of patients leads to VAP [3]. Thereby, decontamination of the mouth and throat using antiseptics is recommended to reduce the aspiration of already existing pathogens in the oral cavity [4]. Based on the guidelines provided by the Centers for Disease Control and Prevention (CDC), staff hygiene and sanitization, use of gloves when dealing with infected patients and in contact with respiratory membrane [5], the elevation of the head of the bed to 30º to 45º, and routine management of oral hygiene for oropharyngeal decontamination using Chlorhexidine Gluconate antiseptic [6]. Chlorhexidine is commonly used in mouthwash to prevent plaque. It is effective against bacteria, viruses, and fungi [7]. It reduces the sticking of bacteria to the oral cavity by increasing the permeability of bacterial cell walls and causing osmotic imbalance [5]. Nonetheless, commonly reported side effects of chlorhexidine, such as tooth discoloration, irritation of the mucous, and mucosal lesions, have raised concerns [6]. Furthermore, studies have also indicated that chlorhexidine might not be effective in reducing the incidence of VAP [8].
Otostegia Persica plant is found in dry tropical and subtropical areas of Iran and is commonly known as Golder [9]. It is a known herbal plant with a number of biological benefits, such as antioxidant, antidiabetic, antimicrobial, and hepatoprotective activities [10]. The ethanol extract of the plant has shown antibacterial effects against a number of gram-positive (Listeria monocytogens, Staphylococcus aureus, and Staphylococcus epidermidis) strains [11]. Several studies have reported that Persica mouthwash has anti-plaque, anti-bleeding, anti-ulcer [12], analgesic and antimicrobial effects with no side effects from chemical mouthwashes [13]. Octacosanol, 1-triacantanol, β-sitosterol, and β-sitosterol-3-O-β-D-glucopyranoside were isolated from the stem of the plant through phytochemical investigations [14]. These active compounds are thought to contribute to the antimicrobial and anti-inflammatory properties of Persica mouthwash, which may help to prevent oral infections, including ventilator-induced pneumonia [15].
The aim of this study is to compare the effects of chlorhexidine and Persica mouthwash in reducing the incidence of VAP and associated outcomes. We also evaluated the side effects of these mouths among ICU patients.
2. METHODS
This double-blind, randomized clinical trial was conducted at the ICU of (Imam Khomeini Hospital in Sari, Iran). The inclusion criteria of the study were patients aged 18-65 years, those intubated for at least 48 hours, and Clinical Pulmonary Infection Score (CPIS) less than 6 (no pneumonia prior to mechanical ventilation). Exclusion criteria of the study were the dissatisfaction of the patient at any time during the study, need for reintubation, autoimmune diseases and malignancies, history of radiotherapy and immunosuppressive drugs such as corticosteroids and immunosuppressive diseases, history of sensitivity to mouthwash, asthma, and allergic rhinitis and dermatitis, history of antibiotic use two weeks before hospitalization, history of using any antimicrobial mouthwash two weeks before hospitalization, oral mucositis and periodontal disease, patients with pneumonia prior to ventilation, pregnancy and history of lung disease (Fig. 1).
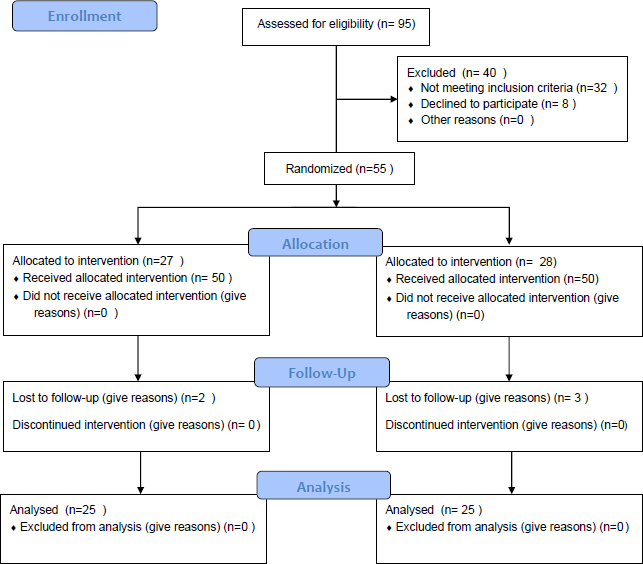
We excluded patients who had a history of pneumonia prior to mechanical ventilation, which includes those who were admitted to the ICU with pneumonia as their primary diagnosis or developed pneumonia during the first 48 hours of intubation. This exclusion criterion ensured that we were able to focus on the effect of the mouthwash intervention on preventing new cases of pneumonia that were acquired during mechanical ventilation. Regarding the other exclusion criteria, we selected them based on existing literature on mouthwash use and potential risk factors for developing complications or adverse reactions. We excluded patients with a history of sensitivity to mouthwash, as they may have experienced adverse reactions to the mouthwash intervention. We also excluded patients with certain medical conditions, such as autoimmune diseases, malignancies, and immunosuppressive diseases, as these conditions may have affected their immune response and increased their risk of developing complications. Additionally, we excluded patients with a history of antibiotic use or use of any antimicrobial mouthwash two weeks before hospitalization, as this may have interfered with the effectiveness of the mouthwash intervention. Finally, we excluded patients with oral mucositis and periodontal disease, as these conditions may have affected the absorption and distribution of the mouthwash, potentially influencing the outcomes of the study.
Patients admitted to ICU under mechanical ventilation were included in the study. According to studies performed by kusahara (34) and considering the incidence of VAP in the control group equal to 67% and in the treatment group equal to 25% (difference equal to 42%) and assuming the possibility of type error equal to 0.05, the study power equal to 80%, the sample size of 25 was calculated.
In this study, 50 patients admitted to the ICU who underwent mechanical ventilation were studied in two groups of 25 each. They were randomly assigned to two groups. The control group used Oral B Soft toothbrush and tongue cleaner twice a day for 3 minutes, followed by 10cc of chlorhexidine 0.2% mouthwash for 6 minutes covering all gingival surfaces of the tongue, throat, and teeth.
In the intervention group, the entire oral cavity of the teeth and tongue was brushed twice a day for 3 minutes with an Oral B Soft toothbrush. The entire gingival surface of the tongue, throat, and tooth surfaces was washed with Persica 10cc mouthwash for 6 minutes.
Persica Porseina mouthwash (Pursina Mouth Wash Persica Drop) is a 100% herbal mouthwash that contains the active ingredients of Salvadora Persica, Mentha Spicata, and Achillea Millefolium.
Patients and researchers involved in obtaining and analyzing the data were not aware of the type of mouthwash. Factors influencing the increase of ventilator-dependent pneumonia such as age, sex, cause of hospitalization in SOFA score (sequential organ failure assessment), number of days of intubation, treatment process (tsinogatnAH2) and history of diabetes were recorded in the data collection form.
Interventions were performed by a fixed ICU staff member. The incidence of mechanical ventilation pneumonia was assessed by CPIS on the third day, and the effect of the two methods on reducing the rate of early ventilator-induced pneumonia was compared. Data for CPIS calculations include variables such as white blood cell count, oxygen uptake (calculated based on Pao2 divided by Fio2), and chest radiography, interpreted by a radiologist. The duration of ventilation, duration of ICU stays, mortality, and side effects were also compared between the two groups. All patients received mechanical ventilation with positive end-expiratory pressure (PEEP). The cuff pressure of the endotracheal tube was checked daily by the relevant measuring device. All patients were in the semi-recumbent position (30°-45°).
Statistical analyses were performed using SPSS v20 software. The normality of values was tested using the Shapiro-Wilk test. To compare the primary and secondary primary outcome variables quantitatively in the two groups, a t-test was used, and in the absence of normal distribution, the Mann-Whitney test was used. The chi-square test or Fisher's exact test was used to compare nominal qualitative variables between the two groups. To investigate the relationships between the main outcome variable and the underlying and baseline variables, the Kendall rank coefficient was used. P-value <0.05 was considered to be significant.
The registration in the International Iranian Clinical Trials Registration Center with IRCT number: RCT2017022032676N1.
This study was approved by the Research Ethics Board of Mazandaran University of Medical Sciences, Sari, Iran (IR.MAZUMS.REC.95.2356).
The methods were stated in accordance with CONSORT criteria.
3. RESULTS
Of 50 patients included in the study, the mean age of patients was 45.04±16.91 years, and their mean body mass index was 27.02±3.71 kg/m2. The mean of days in ICU was 14 days (range: 8-45 days). The average number of days under intubation was 12 days (range: 7-28), and the average number of days under mechanical ventilation was 12 days (range: 6-45).
Data related to body mass index (BMI) and SOFA score had a normal distribution, and other quantitative variables, including age, days of hospitalization in the intensive care unit (ICU), days under intubation, and mechanical ventilation, and CPIS score, had non-normal distribution. Of the 50 patients evaluated in this intervention, 36 patients (72%) were male, and 14 patients (28%) were female (Table 1).
| Variable | Chlorhexidine Treatment | Treatment with Persica | p-value |
|---|---|---|---|
| Age | 44.20 ± 18.35 | 45.88 ± 15.67 | c0.76 |
| BMI | 26.48 ± 3.83 | 3.59 ± 27.56 | d0.30 |
| ICU admit day | 18.32 ± 11.26 | 14.08 ± 5.56 | c0.22 |
| Intub Days | 12.88 ± 5.21 | 11.84 ± 4.11 | c0.59 |
| Venti Days | 16.52 ±11.71 | 12.64 ± 5.39 | c0.48 |
| Sofa score | 7.72 ± 3.14 | 8.80 ± 3.62 | d0.26 |
| Cpis | 4.24 ± 1.71 | 3.92 ± 2.01 | c0.56 |
| Variable | Chlorhexidine Treatment Frequency(%) | Treatment with Persica Frequency (%) | p-value | |
|---|---|---|---|---|
| Gender | Male | 18(72%) | 18(72%) | 1.0 |
| Female | 7(28%) | 7(28%) | ||
| Cause of ICU admission | Trauma | 11(44%) | 10(40%) | 0.91 |
| Neurologic | 2(8%) | 2(8%) | ||
| Respiratory distress | 4(16%) | 6(24%) | ||
| After surgery | 8(32%) | 7(28%) | ||
| Diabetes | - | 3(12%) | 2(8%) | 1.0 |
| Variable | Chlorhexidine Treatment Frequency (%) | Treatment with Persica Frequency (%) | Odd ratio | CI95% | P-value |
|---|---|---|---|---|---|
| Complications | 11 (44.0%) | 2 (8.0%) | 4.04 | 14.18 – 1.10 | 0.008 |
| Pneumonia | 3 (12.0%) | 4 (16.0%) | 0.85 | 1.74 – 0.42 | 1.0 |
| Mortality | 4 (16.0%) | 6 (24.0%) | 1.65 | 6.78 – 0.4 | 0.72 |
Causes of ICU admission were trauma in 21 cases (42%), neurological dysfunction in 4 cases (8%), respiratory failure in 10 cases (20%), and 15 cases (30%) were those of postoperative complications. The mortality rate during this study was 20.0% (10 patients) (Table 2). The incidence of side effects in all patients was 26.0% (13 patients). These side effects included 2 cases (4.0%) of dry mouth, 1 case (2.0%) of hypersensitivity, 6 cases (12.0%) of tooth staining, and 4 cases of (8.0) dry mouth with tooth staining. The incidence of pneumonia based on a CPIS score higher than 6 in all patients on the third day under mechanical ventilation was 14.0% (7 patients). The mean and frequency of the above variables in the two treatment groups are described separately in Tables 1-3. The two groups were not significantly different in terms of gender p=1, the cause of ICU admission, p=0.91, the incidence of diabetes, p=1, mortality p=0.72, BMI p=0.3, age p=0.76, days of admission ICU, ventilation and intubation, p=0.22, p=0.48 and p=0.59, respectively, SOFA score p=0.26 and CPIS p=0.56 and the incidence of pneumonia, p=1. However, the incidence of complications in the control group was 44%, and in the intervention group was 8%, which was significantly different (Table 3).
There was a significant relationship between age as a baseline variable and CPIS score as a primary consequence: the Kendall blister coefficient. p-value = 0.001, 0.347. There was also a significant relationship between age and secondary outcomes, including ICU length of stay and mechanical ventilation duration: Kendall Tau B coefficient 0.231 and 0.243, P = 0.02 and p-value = 0.01, respectively. There was no significant relationship between the CPIS score and the SOFA score. P = 0.93. There was no significant relationship between the patient's body mass index as a background variable and the primary and secondary outcomes of patients P>0.05.
4. DISCUSSION
The present study investigated the effect of Persica herbal mouthwash in reducing the incidence of premature pneumonia caused by VAP in ICU patients compared to conventional chlorhexidine mouthwash. In this study, it was shown that the incidence of pneumonia on the third day based on CPIS in patients using Persica mouthwash was not significantly different from chlorhexidine 0.2% (16% and 12% P = 1.0, respectively). The incidence of mortality, duration in the ICU, and the time of intubation and mechanical ventilation were not different in the two groups. However, the incidence of side effects in using Persica herbal mouthwash was significantly lower than chlorhexidine (8 vs. 44%, P = 0.008).
VAP ventilator pneumonia is associated with increased health costs as well as mortality and morbidity. Previous studies have shown that oral health status affects the progression of VAP, and the increase in dental plaque is a predictor of pneumonia in ICU patients with a low initial CPIS score [16]. A recent systematic review and meta-analysis concluded that among patients undergoing heart surgery [17], preoperative use of chlorhexidine gluconate mouthwash significantly reduces the incidence of postoperative VAP compared to patients who do not receive preoperative mouthwash [18]. They did not report any side effects related to this mouthwash [14]. Clinical studies have also reported the use of chlorhexidine 0.2% in reducing the daily risk of VAP compared to the control group [19]. Similar results were reported in the study by [20]; the use of 0.12% chlorhexidine was significantly effective in reducing the incidence of pneumonia three times compared to the control group [21]. However, in a randomized clinical trial, Fourrier and colleagues found no difference in the use of 0.2% chlorhexidine and placebo in the prevention and reduction of VAP [22]. Seyedalshohadaee et al. also reported no difference between 12% chlorhexidine and normal saline in reducing the incidence of VAP [23].
In recent years, herbal mouthwashes with antimicrobial effects have also been introduced. Herbal mouthwashes are more suitable than chlorhexidine due to their natural composition and compatibility with the body and less risk of poisoning. In a study by Khezri et al., the antibacterial effects of Matrica and Persica herbal mouthwashes were compared with chlorhexidine. The results of the study indicated that herbal mouthwashes are as effective as chlorhexidine in reducing the growth of S. aureus and S. pneumonia in VAP patients [24].
In a double-blind, randomized clinical trial by Taraghi et al., the effects of 0.2% chlorhexidine gluconate and 10% Persica mouthwash were compared among ICU patients against VAP and colonization of associated pathogens. The outcomes of the study showed that compared to normal saline solution, both types of mouthwash reduce the incidence of VAP against S. aureus and S. pneumonia. The authors suggested that owing to the increased resistance of pathogens against chlorhexidine, Persica mouthwash can be an effective alternative. The findings of our study are parallel with this study; however, we did not include a saline or placebo group in our study [25].
CONCLUSION
The findings of our study indicated that the incidence of complications was significantly lesser in the Persica group relative to the Chlorhexidine group. Our study is based on limited sample size, and we don’t report data regarding the resistance and/or sensitivity of strains against these antiseptic mouthwashes. Further studies, including microbiological assessment, can, therefore, add significantly to such research.
The use of Persica herbal mouthwash is as effective as chlorhexidine in reducing the incidence of premature ventilator-induced pneumonia and mortality. However, the herbal mouthwash of Persica has fewer side effects among ICU patients.
ATUHORS' CONTRIBUTIONS
Dr. Farshad Hassanzadeh Kiabi and Dr. Abdolmajid Gholinataj Jelodar: conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript.
Dr. Afshin Gholipour Baradari and Dr.Alieh Zamani Kiasari: Designed the data collection instruments, collected data, carried out the initial analyses, and reviewed and revised the manuscript.
Dr.Mahdi Shahheidari: Coordinated and supervised data collection, and critically reviewed the manuscript for important intellectual content.
LIST OF ABBREVIATIONS
| BMI | = Body Mass Index |
| ICU | = Intensive Care Unit |
| VAP | = Ventilator-Associated Pneumonia |
| CPIS | = Clinical Pulmonary Infection Score |
| SOFA | = Sequential Organ Failure Assessment |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the Research Ethics Board of Mazandaran University of Medical Sciences, Sari, Iran (IR.MAZUMS.REC.95.2356).
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures followed were in accordance with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Informed consent was obtained from each participant.
AVAILABILITY OF DATA AND MATERIALS
All relevant data and materials are provided in the manuscript.
STANDARDS OF REPORTING
CONSORT guidelines were followed.
FUNDING
None.
CONFLICT OF INTEREST
The authors deny any conflict of interest in any terms or by any means during the study.
ACKNOWLEDGEMENTS
Declared none.


