All published articles of this journal are available on ScienceDirect.
Lifestyle Risk Factors Associated with Stroke in Patients with Type 2 Diabetes Mellitus: A Case-control Study
Abstract
Background:
Distinct lifestyle-related cardiovascular risk factors can be present simultaneously in patients with type 2 diabetes mellitus, thus increasing the odds of developing a stroke.
Objective:
The aim of this study was to establish the risk factors associated with the development of stroke in patients with type 2 diabetes mellitus at the Internal Medicine Services of a referral hospital in Peru.
Methods:
A case-control study with an unpaired design was conducted, evaluating 324 patients diagnosed with type 2 diabetes mellitus, 108 having a confirmed diagnosis through computed tomography scan and 216 patients without such confirmation, at a reference hospital in Peru from 2012 and 2021. Clinical and laboratory parameters related to lifestyle were evaluated. Odds ratios with 95% confidence intervals were obtained. Logistic regression was used for multivariate analysis.
Results:
In the multivariate analysis, being 60 years of age or older: ORa 1.04; 95%CI 1.02-1.07, hypertension: ORa 5.26; 95%CI 2.84-9.74, as well as elevated levels of glycated hemoglobin (HbA1c) levels: ORa 1.11; 95% CI 1.00-1.23 and C- reactive protein (CRP) levels ORa 1.04; 95% CI 1.01-1.06) were significantly associated with stroke risk.
Conclusion:
The risk factors associated with the development of strokes in patients diagnosed with type 2 diabetes mellitus were advanced age (over 60 years), chronic inflammation (elevated CRP levels), inadequate metabolic control (elevated HbA1c levels), and, more conclusively, hypertension. These factors are all related to lifestyle, highlighting the importance of promoting proper management in this population.
1. INTRODUCTION
Type 2 diabetes mellitus is a chronic non-communicable disease and represents, like other diseases of this type, a significant burden of morbidity, leading to disability or mortality with devastating consequences for the economies worldwide [1].
As of 2021, there were an estimated 537 million adults affected by this pathology globally; in other words, 10% of the population within this age group. In the United States, the incidence rate is estimated to be around nine new cases per 100 individuals per year, with a higher prevalence among those aged over 65 and above [2]. This proportion becomes even larger when considering low or middle-income countries, where three out of four patients with diabetes live, out of the total worldwide [3]. Specifically, in the Americas, 410,000 deaths were attributed to this pathology during the year 2021 [3]. As a result, it reduces life expectancy by an average of 10 years, with cardiovascular diseases being the leading cause of morbidity and mortality among patients suffering from this pathology [4].
Furthermore, diabetes is known for being a known risk factor for the development of strokes [5]. Indeed, diabetes is associated with several microvascular and macrovascular alterations that often lead to major clinical complications, including strokes [4]. Diabetes is considered a modifiable condition that contributes to the increase in the risk of stroke development. It is even more worrying that mortality in the diabetic population is higher, and the evolution after the event is poorer compared to those without diabetes. In particular, the risk of developing an ischemic stroke is two to three times higher in patients with type 2 diabetes mellitus. This is especially relevant considering the high incidence of stroke, especially of ischemic events, and with a higher proportion in male individuals [6].
The enhanced cardiovascular risk observed in those patients cannot be relieved by the intervention with a single factor for glycemic control but requires multifactorial control of cardiovascular risk factors [4]. Thus, it is crucial to prioritize lifestyle modifications and address other risk factors in order to achieve adequate and effective control [7]. While type 2 diabetes mellitus is a well-defined cardiovascular risk factor, its interaction with other risk factors in relation to stroke risk is the primary reason for focusing on this vulnerable group of patients. This is especially relevant in a population, such as the Peruvian one, where the coexistence of many of these is common, and health often lacks appropriate approaches and treatments, as seen globally [6, 8]. Moreover, there is a lack of substantial evidence regarding lifestyle factors and their association with the development of stroke in Peruvian diabetic patients.
This study was conducted to establish the risk factors associated with the development of a stroke in patients diagnosed with type 2 diabetes mellitus within the internal medicine services of a referral hospital in Peru from 2012 to 2021.
2. MATERIALS AND METHODS
2.2. Selection of Participants
For the cases, patients diagnosed with type 2 diabetes mellitus who were hospitalized in the internal medicine services of a hospital in Peru between 2012 and 2021 were included. Only patients who had the diagnosis of a stroke confirmed through tomography, aged 18 years or older, and with complete medical records were considered. Patients with a diagnosis of type 1 diabetes mellitus, gestational diabetes, and those with incomplete medical records were excluded.
For the controls, patients with a diagnosis of type 2 diabetes mellitus who were hospitalized in the internal medicine services in a hospital in Peru during the same period, without a clinical-radiological diagnosis of a stroke (through computed tomography scan) who were 18 years of age or older and had medical records in which the variables to be evaluated could be accurately determined were considered. Exclusion criteria were medical records of patients not being hospitalized in the specified services during the study period and those not adequately recorded.
Both the case and control groups were selected from the same study population. Controls were randomly selected in an unpaired and simple randomized manner.
2.3. Variables and their Operationalization
The data for both clinical and laboratory variables were taken from the initial evaluations conducted upon hospital admission and recorded in the patients’ medical records at the time of the study event.
The variables considered as risk factors were sociodemographic factors (age greater than or equal to 60 years [9] and male or female gender), history of acute myocardial infarction, the presence of chronic kidney disease (CKD) (defined by a glomerular filtration rate of less than 60 ml/min/1.73 m2), elevated levels of glycated hemoglobin (HbA1c)≥8% (64 mmol/mol) [10] presence of arterial hypertension (HTN) (defined by more than 140 mmHg systolic blood pressure and/or more than 90 mmHg diastolic blood pressure according to the International Society of Hypertension [11], presence of overweight (Body Mass Index (BMI) between 25 to 29.9 kg/m2), obesity (BMI of 30 or more kg/m2), smoking, dyslipidemias (elevated levels of total cholesterol, triglycerides, LDL, and decreased HDL according to Fredrickson’s classification [12]) and elevated levels of C-reactive protein (CRP)) defined by more than 10 mg/dl [13].
2.4. Population and Sample
The population consisted of patients hospitalized in the internal medicine services of a hospital in Peru during the 2012-2021 period.
The sample consisted of 324 patients, of which 108 corresponded to cases and 216 to controls. The sample size was calculated using a formula for unpaired case-control studies, with a frequency of exposure among controls of 50%, based on a study by Ohishi [14]. The predicted Odds Ratio for the HTN variable was 1.96 (95% CI 1.097-3.501, P=0.023) [15]. The confidence level was estimated at 95%, and the number of controls per case was set at two. The sampling method was probabilistic.
2.5. Statistical Analysis
A Microsoft Excel database was created to organize the information, and the statistical analysis was performed using SPSS version 26. Initially, a descriptive analysis was conducted to establish the frequency of presentation of each study, which was variable to, at a later stage, perform data analysis using contingency tables to calculate the Odds Ratio with 95% confidence intervals and the evaluation of the risk relationship between study variables.
For the bivariate inferential analysis, the Chi-square test was applied to analyze qualitative variables and the presence of stroke, taking into consideration the value of p <0.05 as statistically significant. Logistic regression was used for multivariate analysis.
3. RESULTS
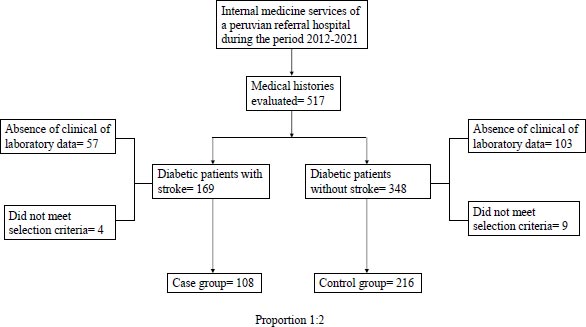
The population corresponding to patients with a diagnosis of type 2 diabetes mellitus was 7,220, of whom 2.13% had a record of stroke. From this population, 108 clinical records were randomly selected, taking a ratio of one case for every two controls; 216 of them were randomly selected, as shown in Fig. (1).
In the case of the controls, the average age was 60.1 years, with a standard deviation of 12.8. On the other hand, among the cases, the average age was 66 years, with a standard deviation of 10.7. The presence of obesity was found in 80.6% of the controls, while it was present in almost 1% more of the cases. In addition, HTN was present in 38% more cases in comparison with controls (Table 1).
| Variables | Categories | Cases | Controls | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | Average | SD | n | % | Average | SD | ||
| Age (years) | ≥60 | 84 | 77.8% | 66,6 | 10,7 | 116 | 53.7% | 60.1 | 12.8 |
| <60 | 24 | 22.2% | 100 | 46.3% | |||||
| Gender | Female | 54 | 50.0% | - | - | 80 | 37.0% | - | - |
| Male | 54 | 50.0% | - | - | 136 | 63.0% | - | - | |
| BMI | Overweight and obesity | 88 | 81.5% | - | - | 174 | 80.6% | - | - |
| Normal weight | 20 | 18.5% | - | - | 42 | 19.4% | - | - | |
| Smoking | Yes | 22 | 20.4% | - | - | 36 | 16.7% | - | - |
| No | 86 | 79.6% | - | - | 180 | 83.3% | - | - | |
| History of acute myocardial infarction | Yes | 16 | 14.8% | - | - | 12 | 5.6% | - | - |
| No | 92 | 85.2% | - | - | 204 | 94.4% | - | - | |
| Presence of CKD | Yes | 21 | 19.4% | - | - | 37 | 17.1% | - | - |
| No | 87 | 80.6% | - | - | 179 | 82.9% | - | - | |
| Presence of HTN | Yes | 86 | 79.6% | - | - | 89 | 41.2% | - | - |
| No | 22 | 20.4% | - | - | 127 | 58.8% | - | - | |
| HbA1c | ≥8% (64 mmol/mol) | 77 | 71.3% | 9.7 | 2.5 | 137 | 63.4% | 9 | 2.6 |
| <8% (64 mmol/mol) | 31 | 28.7% | 79 | 36.6% | |||||
| Total cholesterol (mg/dl) | ≥200 | 15 | 13.9% | 159.6 | 37.5 | 25 | 11.6% | 155.4 | 37.3 |
| <200 | 93 | 86.1% | 191 | 88.4% | |||||
| LDLc (mg/dl) | ≥100 | 59 | 54.6% | 98 | 33.3 | 118 | 54.6% | 97.8 | 28.6 |
| <100 | 49 | 45.4% | 98 | 45.4% | |||||
| HDLc (mg/dl) | <40 | 70 | 64.8% | 37.8 | 9.8 | 141 | 65.3% | 37.3 | 10.9 |
| ≥40 | 38 | 35.2% | 75 | 34.7% | |||||
| Triglycerides (mg/dl) | ≥150 | 65 | 60.2% | 151.2 | 45.8 | 114 | 52.8% | 158.1 | 73.5 |
| <150 | 43 | 39.8% | 102 | 47.2% | |||||
| CRP (mg/dl) | ≥10 | 76 | 70.4% | 13.7 | 13.3 | 104 | 48.1% | 13 | 10.5 |
| <10 | 32 | 29.6% | 112 | 51.9% | |||||
| Total | - | 108 | - | - | - | 216 | - | - | - |
| Variables | Categories | ORc | 95% CI | p | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Age (years) | ≥60 | 3.02 | 1.78 | 5.11 | <0.001 |
| <60 | REF | REF | REF | REF | |
| Gender | Female | 1.70 | 1.07 | 2.71 | 0.26 |
| Male | REF | REF | REF | REF | |
| BMI | Overweight and obesity | 1.06 | 0.59 | 1.92 | 0.84 |
| Normal weight | REF | REF | REF | REF | |
| Smoking | Yes | 1.28 | 0.71 | 2.31 | 0.41 |
| No | REF | REF | REF | REF | |
| History of Acute Myocardial Infarction | Yes | 2.96 | 1.34 | 6.50 | 0.01 |
| No | REF | REF | REF | REF | |
| Presence of CKD | Yes | 1.17 | 0.64 | 2.11 | 0.61 |
| No | REF | REF | REF | REF | |
| Presence of HTN | Yes | 5.58 | 3.25 | 9.58 | <0.001 |
| No | REF | REF | REF | REF | |
| HbA1c | ≥8% (64 mmol/mol) | 1.43 | 0.87 | 2.36 | 0.16 |
| <8% (64 mmol/mol) | REF | REF | REF | REF | |
| Total cholesterol (mg/dl) | ≥200 | 1.23 | 0.62 | 2.45 | 0.55 |
| <200 | REF | REF | REF | REF | |
| LDLc (mg/dl) | ≥100 | 1.00 | 0.63 | 1.59 | 1.00 |
| <100 | REF | REF | REF | REF | |
| HDLc (mg/dl) | <40 | 0.98 | 0.60 | 1.59 | 0.93 |
| ≥40 | REF | REF | REF | REF | |
| Triglycerides (mg/dl) | ≥150 | 1.35 | 0.85 | 2.16 | 0.21 |
| <150 | REF | REF | REF | REF | |
| CRP (mg/dl) | ≥10 | 2.56 | 1.56 | 4.18 | <0.001 |
| <10 | REF | REF | REF | REF | |
| Variables | Categories | ORa | 95%CI for adjusted OR | p | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Age (years) | ≥60 | 1.04 | 1.02 | 1.07 | <0.001 |
| <60 | REF | REF | REF | REF | |
| Gender | Female | 1.36 | 0.79 | 2.37 | 0.27 |
| Male | REF | REF | REF | REF | |
| Overweight | Yes | 0.81 | 0.39 | 1.66 | 0.565 |
| No | REF | REF | REF | REF | |
| Obesity | Yes | 1.99 | 0.84 | 4.76 | 0.120 |
| No | REF | REF | REF | REF | |
| Smoking | Yes | 1.31 | 0.66 | 2.59 | 0.435 |
| No | REF | REF | REF | REF | |
| History of Acute Myocardial Infarction | Yes | 2.02 | 0.81 | 5.04 | 0.131 |
| No | REF | REF | REF | REF | |
| Presence of CKD | Yes | 0.84 | 0.40 | 1.75 | 0.642 |
| No | REF | REF | REF | REF | |
| Presence of HTN | Yes | 5.26 | 2.84 | 9.74 | <0.001 |
| No | REF | REF | REF | REF | |
| HbA1c | ≥8% (64 mmol/mol) | 1.11 | 1.00 | 1.23 | 0.050 |
| <8% (64 mmol/mol) | REF | REF | REF | REF | |
| Total cholesterol (mg/dl) | ≥200 | 1.01 | 1.00 | 1.02 | 0.104 |
| <200 | REF | REF | REF | REF | |
| LDLc (mg/dl) | ≥100 | 1.00 | 0.98 | 1.01 | 0.636 |
| <100 | REF | REF | REF | REF | |
| HDLc (mg/dl) | <40 | 0.98 | 0.95 | 1.01 | 0.286 |
| ≥40 | REF | REF | REF | REF | |
| Triglycerides (mg/dl) | ≥150 | 1.00 | 0.99 | 1.00 | 0.048 |
| <150 | REF | REF | REF | REF | |
| CRP (mg/dl) | ≥10 | 1.04 | 1.01 | 1.06 | 0.002 |
| <10 | REF | REF | REF | REF | |
In the bivariate analysis, it was found that being 60 years of age or older (ORc 1.05; 95%CI 1.03-1.07; p =0.001), history of acute myocardial infarction (ORc 2.96; 95%CI 1.35-6.50; p=0.01), presence of HTN (ORc 5.58; 95%CI 3.25-9.58; p <=0.001) and elevated CRP levels (ORc 1.03; 95%CI 1.01-1.05; p =0.001) showed a significant association (Table 2).
Subsequently, in the multivariate analysis using logistic regression, the following variables were found to show statistical significance: age equal to or greater than 60 years (ORa 1.04; 95%CI 1.02-1.07; p = 0.001), presence of HTN (ORa 5.26; 95%CI 2.84-9.74; p = 0.001), elevated glycated hemoglobin levels (ORa 1.11; 95%CI 1.00-1.23; p = 0.05) and elevated CRP (ORa 1.11; 95%CI 1.00-1.23; p = 0.05). 05) and CRP (ORa 1.04; 95%CI 1.01-1.06; p = 0.002) (Table 3).
4. DISCUSSION
The present investigation contributes to the existing national evidence, as well as to research in other developing countries concerning the factors linked to cardiovascular disease development in individuals with diabetes, with a particular focus on lifestyle-related factors. The findings reveal that at 60 years of age or older, the presence of arterial hypertension, and elevated CRP and HbA1c levels were significantly associated with the development of stroke in diabetic patients in a public referral hospital in Peru.
Strokes have negative consequences at the individual, family, and social levels and represent a major public health problem in both developed and developing countries [7]. Thus, in Latin America, both the incidence of type 2 diabetes mellitus and stroke are high [6, 16]. This indicates the need to emphasize prevention, especially in the diabetic population. In this sense, the importance of analyzing and determining risk factors would provide evidence for their recognition and control in clinical practice.
Improving stroke outcomes in people with diabetes requires not only drug therapy approaches but also the adoption of beneficial lifestyle practices [4] and control of risk conditions, taking into account that the presence of one or more of them increases the risk of the development of this neurological complication [17, 18].
4.1. Aging
According to the data analyzed, 60 years of age or older was identified as a risk factor for developing strokes in diabetic patients. This is supported by another case-control study conducted in the general population, which found that being 60 years of age or older carried the same risk [19]. Thus, it can be stated that older age is generally associated with a higher risk of strokes in the general population without type 2 diabetes mellitus. In diabetic patients, this age difference is attenuated, suggesting that the risk of strokes in people with diabetes may extend even to individuals younger than 60 years, as found by Yen [20].
Furthermore, in a review article evaluating the epidemiology of neurological pathology in diabetic patients, it was stated that the risk of developing it is higher for men between the fifth and sixth decade of their life and women during their sixth decade, which aligns with the results of this study [21].
The pathophysiological mechanism of aging may be due to the alterations suffered by the cerebral circulation in the elderly population (such as increased arterial stiffness and endothelial dysfunction), which increases susceptibility to vascular insufficiency and ischemic injury [22, 23]. Additionally, diabetes promotes a state of cellular senescence that can lead to lipotoxicity and tissue dysfunction, as well as chronic inflammation and degradation of the cellular matrix, which is directly linked to atherosclerosis [24].
4.2. Hypertension
It is essential to highlight that in this study, the presence of arterial hypertension was the factor that increased the risk of developing a stroke in diabetic patients by more than 5 times. Likewise, a cross-sectional study with more than 11,000 diabetic individuals found a similar result (OR 2.82; 95%CI 0.29-17.3) [25]. Furthermore, Berenguer et al. [19] found that HTN is associated with the development of such neurological pathology in the general population (OR 6.6; 95% CI 3.05- 14.41).
Lifestyle is strongly related to blood pressure values due to several factors: high salt intake, smoking, reduced physical activity, and an inadequate diet that can lead to overweight, obesity, and of course, type 2 diabetes mellitus [26, 27].
Additionally, it has been suggested that HTN plays a leading role in the promotion of oxidative stress via the production of reactive oxygen species, the dysfunction of baroreceptors, making them less sensitive to their excitatory stimuli (the increase in blood pressure), thus promoting inflammation, via molecules such as CRP, interleukins 1 and 6, leukocyte esterase and intracellular adhesion molecule-1, and thus leading to functional changes of the cerebral vasculature, which generate hypoperfusion and eventually, ischemia [28].
Among the vascular changes associated with hypertension is the deterioration of cerebral blood flow autoregulation, a mechanism that normally allows cerebral blood flow to maintain a stable perfusion pressure despite variations in systemic pressure. In addition, one of the most important consequences of HTN is its effect on cerebral arteries and arterioles, leading to vascular remodeling, increasing their tone and decreasing their lumen, which increases the severity and extent of the penumbra zone during a stroke [29].
On the other hand, HTN provides potent vasoconstrictor stimuli, such as angiotensin II, which acts via its AT1 receptors and causes deleterious effects at different levels; endothelin-1, which, added to the nitric oxide deficit, also induced by HTN, promotes an intense increase in vascular tone; and the activation of endothelial calcium channels and in vascular smooth muscle [29].
4.3. Chronic Inflammation
The presence of elevated C-reactive protein levels in diabetic patients slightly increases the risk of developing a stroke. This is supported by the findings of a case-control study, where elevated CRP levels were found to increase the risk of developing a stroke in diabetic patients (OR 2.61; 95%CI 2.31-6.42) and in patients without such pathology (OR 2.41; 95%CI 1.93-4.21) [30]. This is in addition to the findings of The Emerging Risks Factors Collaboration consortium, which analyzed over 130 prospective studies [31] and concluded that elevated CRP levels had a relative risk (RR) of 1.46 for the development of ischemic stroke.
Precisely, the role of inflammation lies in its behavior as a precipitating factor of increased blood viscosity, an atherogenic factor in all its phases, both by CRP and by tumor necrosis factor-alpha and interleukin 6 [32]. In response to these stressors, the vascular endothelium produces cell adhesion proteins that facilitate leukocyte adhesion, while tissue macrophages increase the release of cytokines. These molecules will then stimulate the migration of muscle cells from the adventitia to the other arterial layers to form the fibrous cap of the atheromatous plaque, the elementary lesion of atherosclerosis [33].
4.4. Inadequate Metabolic Control
Elevated HbA1c levels were found to be a risk factor in diabetic patients for the development of strokes in diabetic patients [34]. In the same sense, a cross-sectional analytical study in hospitalized diabetic patients found that individuals with elevated HbA1c levels were six times more likely to develop such a neurological event in diabetic patients (OR 6.29; 95%CI 1.71-23) [35]. In addition, a meta-analysis and systematic review concluded that increased levels of this molecule were associated with the development of the event in patients with the same endocrine pathology (HR 1.19; 95%CI 0.87-1.62) [10]. Likewise, it was found in a cohort study with more than 270,000 diabetic patients. It was found that HbA1c above its target values was the strongest predictor for the development of strokes in diabetic patients [36].
HbA1c is used as an assessment of glycemic control from 8 to 12 weeks before measurement [37]. Therefore, its elevation increases the risk of developing both microvascular and macrovascular complications related to diabetes mellitus [35]. This is relatively frequent since, for instance, in the United States [38] and some Latin American countries [39], these increased HbA1c levels are present in more than 20% of the diabetic population.
On the other hand, the variables studied that did not reach significance after multivariate analysis were dyslipidemia, history of acute myocardial infarction, presence of CKD, overweight, obesity, and smoking. The lack of significance in the case of smoking may be attributed to the fact that stroke had the lowest percentage of events attributable to smoking in the population of the region studied [40]. Regarding the lack of significant association of obesity, it may be because diabetic patients with normal weight could be metabolically obese (i.e., the result of the interaction between hyperinsulinemia, insulin resistance and dyslipidemia) or have insufficient insulin secretion as a result of the progression of the disease, which can lead to catabolism [41].
5. LIMITATIONS
This study was limited by the fact that it was carried out with data from a single institution. In addition, given that the study was retrospective, it was impossible to study other variables, such as weight loss or gain. Nevertheless, the sample studied corresponds to that of a national reference hospital, thus providing evidence that should be supported by multicenter or prospective studies.
CONCLUSION
The risk factors associated with the development of strokes in patients with a type 2 diabetes mellitus diagnosis were advanced age (over 60 years), chronic inflammation (increased CRP levels), inappropriate metabolic control (elevated HbA1c levels) and, particularly, hypertension. Therefore, it is recommended to include the evaluation of these lifestyle factors in clinical practice, as well as to consider their importance in the prevention and treatment of stroke in diabetic patients.
As previously mentioned, there is a need for additional national evidence regarding lifestyle-related factors, as they play a crucial role in the preventive approach to chronic non-communicable diseases. We consider that future research should focus on the study of each risk factor in detail, with particular emphasis on unhealthy habits, since there is limited evidence within the regional context.
AUTHORS' CONTRIBUTIONS
All authors made substantial contributions to the conception and design of the study. Alvaro Oyarce-Calderón and Jhony A. De La Cruz-Vargas conducted the material preparation, data collection, and data analysis. The first draft of the manuscript was written by Alvaro Oyarce-Calderón. All authors read and approved the final manuscript.
LIST OF ABBREVIATIONS
| CKD | = Chronic kidney disease |
| HbA1c | = Glycated hemoglobin |
| HTN | = Arterial hypertension |
| BMI | = Body mass index |
| CRP | = C-reactive protein |
| RR | = Relative risk |
| CKD | = Chronic kidney disease |
| ORc | = Crude odds ratio |
| CI | = Confidence interval |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The development of this study was endorsed by the ethics committee of a Peruvian National Hospital (by letter No 0208-2021-DG-HNDM) and by a Peruvian University (by electronic communication No 2373-2021-FMH-D).
HUMAN AND ANIMAL RIGHTS
The standards for research involving human subjects complied with the principles established in the Declaration of Helsinki, ensuring the confidentiality and privacy of the collected data at all times. In addition, all participants provided informed consent prior to their inclusion in the study. This research did not involve the use of animals.
CONSENT FOR PUBLICATION
Informed consent was obtained from all participants.
STANDARDS OF REPORTING
STROBE guidelines were followed.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article are available from the Institutional Repository of Ricardo Palma University at https://hdl.handle.net/20.500.14138/6215.
The dataset(s) supporting the conclusions of this article is(are) available at the following link:
https://docs.google.com/spread
sheets/d/1vLmCWTXh2QuRS9dDlKmvvdcoTo06dGVu/edit?usp=share_link&ouid=102636949378428760472&rtpof=true&sd=true
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


