All published articles of this journal are available on ScienceDirect.
Evaluation of Adverse Effects after the Second Dose of BNT162b2 Mrna Vaccination for COVID-19: A Survey-based Analysis among Italian Healthcare Workers
Abstract
Backgrounds and Aims:
Since the start of the SARS-CoV-2 pandemic, healthcare workers have been at elevated risk of contracting COVID-19. Although COVID-19 vaccines have contributed to the eradication of, or substantial decreases in, the incidence of lethal diseases, the major determinant of COVID-19 vaccine hesitancy is a fear of associated adverse effects. Here, we performed a survey assessing the reactogenicity and safety of BNT162b2 in a real-world setting.
Methods:
Data were collected from March 1 and June 14, 2021. A total of 206 hospital employees undergoing BNT162b2 mRNA vaccination completed the survey. These hospital workers received a questionnaire to collect the common and uncommon adverse effects developing 2–6 days after the second dose of the Pfizer-BioNTech vaccine.
Results:
After the second dose, female sex was found to be associated with a higher risk of vaccine-related severe systemic adverse effects than male sex (odds ratio [OR] 3.116, 95% CI 2.365–7.113). We also observed that the anti-SARS-CoV-2 receptor-binding domain titer, determined on the day when the second dose of the Pfizer-BioNTech vaccine was administered, was significantly higher in participants with severe systemic effects than those without such effects (OR 1.017, 95% CI 1.001–1.034).
Conclusion:
Our study suggested that healthy female healthcare workers had a three-fold higher risk than healthy male healthcare workers of developing severe adverse effects after the second dose of the Pfizer-BioNTech vaccine. Further research is warranted to determine whether a high anti-SARS-CoV-2 RBD titer determined at the time of the second vaccination might indicate a disproportionate inflammatory systemic reaction leading to severe adverse effects. Our findings might contribute to a decrease in the disappearance of COVID-19 vaccine hesitancy.
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and the resulting coronavirus disease 2019 (COVID-19) has affected tens of millions of people in a worldwide pandemic [1]. Vaccines are important to prevent COVID-19, protect people at high risk of complications and ultimately end the pandemic. In this connection, it has been recently reported that more than 14 million deaths were averted through COVID-19 vaccination, a finding that further emphasizes the benefits of the COVID-19 vaccines [2].
The BNT162b2 vaccine is a lipid nanoparticle– encapsulated, nucleoside-modified RNA that encodes a version of the SARS-CoV-2 spike protein with mutations that lock the protein into a conformation that induces neutralizing antibody responses [3]. BNT162b2 vaccine protection against COVID-19 begins approximately 2 weeks after the first vaccine dose [4, 5]. As reported in phase 3 trials, the BNT162b2 vaccine has an efficacy of 52% at 12 days after the first dose and of 95% after the second dose if doses are administered 3–4 weeks apart in people without prior SARS-CoV-2 infection [6]. However, only modest neutralization activity of sera is observed shortly before the second vaccine administration, and a second dose is required to obtain a robust increase in neutralizing antibody titers [7, 8].
Vaccine safety is critical for successfully implementing any vaccination program, particularly during a pandemic. Accordingly, phase 3 clinical trials have indicated that COVID-19 vaccines have an acceptable safety profile. Notably, in clinical trials of the BNT162b2 vaccine, the participants had a low incidence of serious adverse local and systemic reactions [9-13]. However, the Pfizer/BioNTech vaccine has been reported to cause mild adverse effects after the first dose but a greater rate of adverse effects after the second dose [14]. A recent systematic review and an observational cross-sectional study conducted among the healthcare worker (HCW) population in Ecuador have found that side effects after the Pfizer-BioNTech vaccine are common, but they are usually mild and self-limited [15, 16].
The reported adverse events include pain, redness or swelling at the injection site, fever, fatigue, headache, muscle pain, nausea, vomiting, itching, chills, joint pain and rarely anaphylactic shock.
Although the Italian Government issued Decree Law no. 44, establishing compulsory COVID-19 vaccination for healthcare workers (HCWs) on April 1, 2021, clinical surveillance remains critical to ensure safety and maintain trust, particularly among hospital personnel. Although COVID-19 vaccines have contributed to the substantial decrease in COVID-19 mortality, the major determinant of COVID-19 vaccine hesitancy is a fear of adverse effects associated with COVID-19 vaccines. However, whether some clinical or biochemical characteristics might influence the rate and severity of adverse effects remains unclear. Therefore, we performed an observational, hospital-based survey conducted by the Tor Vergata occupational medicine service from March 1 and June 14, 2021, to explore the reactogenicity and safety of BNT162b2 in a real-world setting. This study's results may support improving vaccine coverage by preventing vaccine hesitancy in the COVID-19 pandemic.
2. MATERIALS AND METHODS
The study was approved by the Ethics Committee of Polyclinic Tor Vergata, Rome (198/2021). All study participants provided written informed consent before enrollment in the study. The vaccination campaign for HCWs at Polyclinic Tor Vergata, Rome, began on December 28, 2020, and the vaccine used was Pfizer-BioNTech BNT162b2, which is administered intramuscularly in a series of two doses (0.3 mL each) 3 weeks apart. Eight hundred thirty-four hospital HCWs were scheduled for vaccination with two doses of mRNA-BNT162b2 until May 10, 2021. In accordance with protocol, we excluded HCWs with previous SARS-CoV-2 infection, defined by anti-spike positivity before vaccination and/or a history of positive nasopharyngeal swab polymerase chain reaction results. Individuals with any chronic cardio-metabolic diseases, such as arterial hypertension, diabetes, hypercholesterolemia, heart failure, chronic kidney or liver diseases, and any overall systemic chronic diseases requiring continued use of medications that may modulate humoral and cellular immune response (n=72) were also excluded [17]. The remaining 762 HCWs were invited to participate in this observational study on the day of the first vaccination. Respect to excluded HCWs individuals invited to participate did not show any significant differences in terms of age and sex.
On the day of the second Pfizer-BioNTech vaccine dose, blood was drawn from participants to determine glucose, 25-OH vitamin D (Vit D), hemoglobin, white blood cells, serum creatinine and total cholesterol. Vit D was measured with a chemiluminescent microparticle immunoassay (CMIA) test with an Architect Plus I2000 (Abbott©, Chicago, IL, USA) instrument. Total levels of immunoglobulin against the receptor binding domain (RBD) of the SARS-CoV-2 spike protein were assessed with an anti–SARS-CoV-2 S enzyme immunoassay (Roche “Elecsys®” kit) designed for in vitro quantitative determination of antibody levels. The reference value for positivity was > 10 AU /ml.
During the same visit, the Occupational Health Department collected clinical and anthropometric data from study participants, such as their nationality, sex, age, smoking status, education level and BMI. The BMI was calculated as the weight (in kilograms) divided by the square of the height (in meters). The average of three measurements was used to calculate systolic and diastolic blood pressure. Current and former smokers were grouped into a single cluster and compared with participants who never smoked. Participants were asked to complete a questionnaire on the adverse effects developing 2–6 days after the second dose of the Pfizer-BioNTech vaccine. This questionnaire is a modified version of a mandatory document (https://www.aifa.gov.it/moduli- segnalazione-reazioni-avverse) used by the Italian Drug Agency as the COVID-19 campaign began in January 2021 among Italian HCWs.
Completed questionnaires were returned by mail within 1 week after the second vaccine dose. Regarding adverse effects, participants were asked to choose their symptoms from a list of symptoms experienced after vaccination. According to the protocol, we considered the following reported local and systemic reactions in mild to moderate symptoms [4, 18-20]: pain, redness and swelling at the injection site; fatigue; headache; chills; abdominal pain; nausea and sleep disorders [21] including insomnia; fever < 39°C; lymphadenopathy; myalgia/arthralgia; local urticaria; vomiting; and diarrhea. We also considered as severe symptoms the following systemic reactions: dyspnea, paresthesia or fever ≥ 39°C.
The participants were not given any incentives to participate in the study. Nonetheless, the importance of the research in educating the community about the vaccine’s adverse effects was clarified to participants.
2.1. Statistical Analysis
The primary hypothesis of this survey was that equivalent clinical, biochemical and serological characteristics would be observed in participants independently of symptom severity after the second dose of the Pfizer-BioNTech BNT162b2 vaccine. Continuous data were tested for skewness via visual inspection of QeQ plots, stem and leaf plots, or box plots, as well as with the Shapiro-Wilk test for normal distributions. As a consequence, quantitative data were reported as mean ± standard deviation, whereas categorical variables were reported as the number (percentage) of participants. The frequency of local or systemic adverse effects reported after the first and second dose of the BNT162b2 vaccine was evaluated by the exact X2 test.
Correlation coefficients were used to describe bivariate relationships between variables (Pearson correlation coefficient or Spearman's rank correlation coefficient, as appropriate).
A minimum number of 108 subjects will guarantee a power of 95%, assuming a standard deviation of 0.5 (with a total planned type I error rate of 5%), in detecting the presence of severe symptoms in our study group.
In our initial statistical analysis plan, age, sex, job tasks, BMI, systolic and diastolic BP, fasting glucose, total cholesterol, Vitamin D, white blood cells, Hgb, creatinine, Smoking and Anti-SARS-CoV-2 RBD (21 days) were systematically forced in the models. However, because most variables did not contribute to the risk of the primary outcome, our logistic regression model ultimately took into account only serologic testing and sex. We also forced into the model BMI and age, although not correlated to the dependent variable, because of their clinical and biological meaning.
All tests were two-sided, with statistical significance set at p ≤ 0.05. All analyses were performed in SPSS version 19.0 for Windows.
3. RESULTS
We included a group of volunteer hospital workers (n=216, 93 men and 123 women, between 30 and 55 years of age) who agreed to complete the questionnaire and return it by mail within 7 days after the second dose of the Pfizer-BioNTech BNT162b2 vaccine. However, ten (4.6%) participants were excluded from the final analysis because of incomplete clinical or anthropometric data. Consequently, this survey was restricted to 206 participants. Among them, we did not observe any serious symptoms consistent with cardiovascular outcomes, such as stroke, myocarditis/pericarditis; acute myocardial infarction; pulmonary embolism; thrombosis with thrombocytopenia syndrome; or serious neurologic events, including Bell palsy, cerebral venous sinus thrombosis and Guillain-Barré syndrome. Notably, only three participants reported close contact with an infected family member during the seven days after the second dose of the Pfizer-BioNTech BNT162b2 vaccine. Table 1 displays the frequency of selected local or systemic adverse effects after the first and second doses of the BNT162b2 mRNA vaccine given as a percentage of participants. As shown, we found some differences in the frequency and duration of local or systemic adverse effects. Fever ≥ 39°C, fatigue and headache were reported as more frequent after the second dose than after the first dose of BNT162b2 mRNA vaccine (p<0.001, p=0.045, and p=0.012 respectively).
| - | AE Reported after the First Dose | AE Reported after the Second Dose | - | ||
|---|---|---|---|---|---|
| Specific Symptoms | Number (n [yes], %) | Duration (more than 4 h, %) | Number (n [yes], %) | Duration (more than 4 h, %) | p |
| Pain | 11, 5.3 | 9, 81.8 | 13, 6.3 | 12, 92.3 | 0.673 |
| Redness | 5, 2.4 | 5, 100.0 | 4, 1.9 | 4, 100.0 | 0.736 |
| Swelling at the injection site | 2, 1.0 | 2, 100.0 | 0, 0.0 | n.a. | n.p. |
| Fatigue | 14, 7.2 | 14, 100.0 | 26, 12.6 | 24, 92.3 | 0.045 |
| Headache | 10, 9.7 | 9, 90.0 | 24, 11.6 | 22, 91.7 | 0.012 |
| Chills | 14, 6.8 | 4, 26.6 | 14, 6.8 | 7, 33.3 | n.p. |
| Abdominal pain and/or nausea | 15, 7.2 | 2, 9.5 | 21, 8.1 | 2, 9.5 | 0.295 |
| Sleep disorders (including insomnia) | 0, 0.0 | n.a. | 1, 0.5 | 1, 100.0 | n.p. |
| Fever ≤ 39 | 24, 11.6 | 24, 100 | 26, 12.6 | 24, 92.3 | 0.762 |
| Lymphadenopathy | 0, 0 | n.a. | 2, 1.0 | 2, 100.0 | n.p. |
| Myalgia and/or Arthralgia | 53, 25.7 | 43, 81.1 | 49, 23.8 | 47, 96.0 | 0.647 |
| Local urticaria | 0, 0.0 | n.a. | 1, 0.5 | 1, 100.0 | n.p. |
| Vomiting and/or diarrhea | 3, 1.4 | 0, 0.0 | 2, 1.0 | 1, 50.0 | 0.654 |
| Dyspnea | 2, 1.0 | 0, 0.0 | 7, 3.4 | 0, 0.0 | 0.091 |
| Paresthesia | 0, 0.0 | 0, 0.0 | 3, 1.5 | 0, 0.0 | n.p. |
| Fever ≥ 39°C | 7, 3.4 | 7, 100.0 | 25, 12.1 | 6, 24.0 | <0.001 |
Table 2.
| - | No Symptoms (n=77) | Mild to Moderate Symptoms* (n=94) | Severe Symptoms** (n=35) | p |
|---|---|---|---|---|
| Age (years) | 43.3±10.9 | 43.1±10.2 | 41.7±8.0 | 0.729 |
| Sex (male/female, %) | 43/34 (56/44) | 42/52 (45/55) | 7/28 (20/80) | 0.007 |
| Job tasks (nurses/others, %) | 57/20 (74/26) | 69/25 (73/27) | 27/8 (77/23 | 0.642 |
| BMI | 23.4±3.4 | 23.6±3.4 | 23.5±3.4 | 0.883 |
| Systolic BP (mmHg) | 121.3±11.2 | 119.7±12.1 | 118.8±12.5 | 0.238 |
| Diastolic BP (mmHg) | 79.5±7.3 | 80.0±9.4 | 80.1±6.8 | 0.781 |
| Fasting glucose (mg/dl) | 90.4±10.4 | 93.7±7.9 | 89.4±9.2 | 0.084 |
| Total cholesterol (mg/dl) | 182.0±24.0 | 196.3±36.9 | 186.5±17.8 | 0.060 |
| Vitamin D (ng/ml) | 29.6±12.6 | 33.7±12.3 | 37.3±13.3 | 0.061 |
| White blood cells (n/mm3) | 6.8±1.6 | 6.5±1.7 | 6.1±1.2 | 0.158 |
| Hgb (gr/dl) | 14.1±1.3 | 14.3±1.3 | 13.9±1.1 | 0.389 |
| Creatinine (mg/dl) | 0.85±0.15 | 0.87±0.18 | 0.84±0.14 | 0.736 |
| Smoking (never vs current/former; %) | 58/19 (75/25) | 68/26 (72/28) | 27/8 (77/23) | 0.782 |
| Anti-SARS-CoV-2 RBD (21 days) | 27.1±18.4 | 29.9±19.2 | 34.8±21.5 | 0.046 |
Abbreviations: BMI, body mass index; BP, blood pressure; Hgb, hemoglobin; RBD, receptor-binding domain; *mild to moderate symptoms include pain, redness and swelling at the injection site; fatigue; headache; chills; abdominal pain; nausea and sleep disorders including insomnia; fever < 39°C; lymphadenopathy; myalgia/arthralgia; local urticaria; vomiting; and diarrhea. **Severe symptoms include dyspnea; paresthesia and/or fever ≥ 39°C
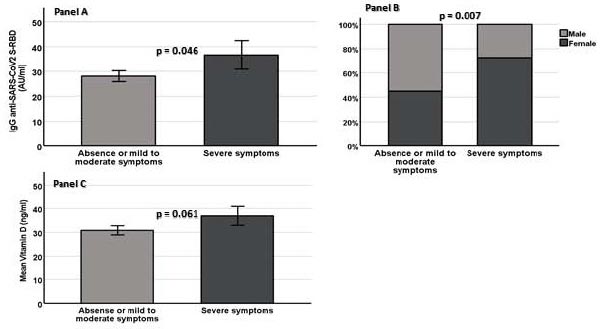
Footnotes: Panel A = IgG anti-Sars-CoV-2 tiber in study population according to intensity of symptoms after second dose of COVID-19 BNT162b2 vaccination; Panel B - Distribution of sex with regard to intensity of symptoms after second dose of COVID-19 BNT162b2 vaccination; Panel C = Vitamin D levels in study population according to intensity of symptoms after second dose od COVID-19 BNT162b2 vacccination.
Table 2 shows the main characteristics of the study population, separated according to the severity of reported symptoms (absence of symptoms, mild to moderate symptoms and severe symptoms) after the second dose of the BNT162b2 vaccine. Most participants reported no symptoms (n=77) or mild to moderate symptoms (n=94). Severe symptoms were reported by 35 HCWs. Notably, we also found a strong, albeit not a statistically significant, direct association between the number of adverse effects and antibody titer either in all study participants or in the 35 HCWs with severe symptoms (Spearman rho=0.123, p=0.078 and Spearman rho=0.116, p=0.117, respectively). However, by dividing the study participants according to the post-vaccination antibody response rate (responsive vs. unresponsive participants, we did find no significant differences in study covariates.
Individuals with severe symptoms were more frequently women (p=0.007) and more likely to have higher 21-day anti-SARS-CoV-2 RBD titers (p=0.046) (Table 2, and Fig. 1, Panel A and Panel B, respectively), whereas all three groups did not significantly differ concerning BMI, systolic and diastolic blood pressure, smoke, sex, glucose, hemoglobin, white blood cell count and numbers of smokers. Similarly, when study participants reported symptoms after the second dose of the BNT162b2 vaccine were compared to individuals without symptoms, the female sex had a significantly higher likelihood of reporting symptoms. Notably, we observed an insignificant increase in Vit D levels in participants with severe symptoms (p=0.061) (Fig. 1, Panel C). Furthermore, dividing HCWs by sex, we observed that with respect to men female participants had significantly higher antibody responses 21 days after the first dose but lower levels of fasting glucose and creatinine.
Interestingly, female sex and 21-day anti-SARS-CoV-2 RBD titer were significantly correlated (Spearman Rho=0.307, p=0.012). For further analysis, we grouped participants with no symptoms or with mild to moderate symptoms. Accordingly, in a regression model adjusted for age, sex, and BMI, 21-day anti-SARS-CoV-2 RBD titer and female sex were significantly associated with severe symptoms independently of other study covariates (Table 3). Similarly, female sex emerged as the only covariate significantly associated with the presence of symptoms (OR=3.115, 95%CI for OR 2.441–7.919, p=0.001) if participants with any type of severity of reported symptoms were grouped and compared to all the other HCWs.
| - | OR | 95% CI for OR | p |
|---|---|---|---|
| Age | 1.003 | 0.966–1.041 | 0.364 |
| Sex (female) | 3.116 | 2.365–7.113 | 0.005 |
| BMI | 1.050 | 0.948–1.163 | 0.770 |
| Anti-SARS-Cov-2 RBD (21 days) | 1.017 | 1.001–1.034 | 0.044 |
In particular, female sex was associated with a markedly greater risk of severe symptoms after the second dose of the Pfizer-BioNTech BNT162b2 vaccine, with a 3.116 estimated OR (95% CI 2.365–7.113, p=0.005). Interestingly, the 21-day anti-SARS-CoV-2 RBD titer remained significantly associated with the severe symptoms, even after adjustment (OR 1.017, 95% CI 1.001–1.034, p=0.044).
4. DISCUSSION
The COVID-19 pandemic has greatly influenced health, economics and society worldwide. Although vaccines have contributed to the eradication of, or substantial decreases in, the incidence of lethal diseases, COVID-19 vaccine hesitancy still poses a great challenge for public health service activities. A major determinant of COVID-19 vaccine hesitancy is a fear of adverse effects associated with the COVID-19 vaccine [22, 23]. Nevertheless, multiple peer-reviewed published studies have shown that the benefits of COVID-19 vaccination outweigh its risks. While some COVID-19 vaccines are associated with a risk of heart inflammation while others are associated with a risk of blood clots, the same risks are much higher following SARS-CoV-2 infection. The available data suggest that the incidence rate of myocarditis in the context of COVID-19 is much greater than the risk of this side effect following vaccination; the benefit of vaccination against COVID-19 outweighs the potential risk of myocarditis and pericarditis in both adolescents and adults [23]. Adverse reactions to the Pfizer-BioNTech COVID-19 vaccine are generally mild and self-limiting [15].
Accordingly, a very recent report used mathematical modeling to estimate the number of deaths averted by COVID-19 vaccination in the first year after the first COVID-19 vaccine was administered post-trial (between 8 December 2020 and 8 December 2021). The researchers estimated that more than 14 million deaths were averted through COVID-19 vaccination, a finding that further emphasizes the benefits of the COVID-19 vaccines [2]. Overall, these recent reports and an information campaign based on sound scientific basis, are supposed to decrease up to the disappearance of COVID-19 vaccine hesitancy.
In this report, we analyzed the profiles of adverse effects after the second dose of the Pfizer-BioNTech BNT162b2 vaccine among HCWs at Polyclinic Tor Vergata. We chose to limit our investigation to the second dose because, during mass vaccination campaigns, findings regarding the safety profiles indicated that people experience more adverse events after the second dose of the BNT162b2 vaccine than the first [14].
Accordingly, our results confirm that the severity of adverse effects after the first BNT162b2 vaccine is lower than after the second dose. In particular, we found that headache, fatigue and fever ≥ 39°C were statistically less frequent (Table 1) and tended to be higher after the second dose.
We observed that most study participants developed mild to moderate reactions after the second BNT162b2 vaccination, by previous study findings. In particular, a study conducted in the UK, which analyzed data reported by people who used the COVID Symptom Study app, has reported that headache, fatigue, pain and tenderness at the injection site are the most common adverse effects [6]. However, on the basis of our results, vaccine recipients may also expect to have no symptoms during the post-vaccination period. This result was not unexpected because the participants in this study were rather young, active hospital employees without any serious chronic diseases (no pharmacological therapy). Even if a clear mechanism is unclear, we cannot exclude the possibility that these characteristics might have increased the tolerability of minor annoyances after BNT162b2 vaccination among our cohort.
On the other hand, the rate of the reported severe symptoms seems remarkably high (almost 17% of participants). Notably, most of the severe adverse events reported after the BNT162b1 second dose (25 out of 35 participants, 71%) were fever ≥ 39°C. The design of this study does not allow for generating any clear hypothesis to explain this finding. However, it is possible that the study’s use of self-reported symptoms after vaccination rather than individual clinical consultation of the participants might have resulted in bias.
The main study result indicated a preponderance of severe adverse effects among women who also showed a significantly higher antibody response 21 days after the first dose but lower levels of fasting glucose and creatinine. This finding deserves to be further investigated in larger studies, possibly revealing a relation among metabolism, kidney function and immune response in HCWs.
Overall, our result is in agreement with previous investigations showing that women are more likely than men to report adverse events after BNT162b2 vaccination [25, 26]. Although the pathophysiological mechanisms underlying the high-intensity development of adverse effects after vaccination in women remain unclear, women exhibit greater humoral and cell-mediated immune responses than men after antigenic stimulation, including vaccination and infection [10]. Accordingly, women show a more pronounced immune response than men after being vaccinated with seasonal influenza [27]. This finding may be due to the greater number of absolute of CD4-positive (CD41) lymphocytes in women than men [28]. Similarly, women have greater production of TH1 cytokines [29, 30], thereby resulting in higher postvaccination release of neutralizing antibodies. Therefore, we speculate that our results might be associated with opposing hormonal effects in the adaptive and innate immune system, in which elevated estradiol increases, and testosterone decreases, vaccine-induced antibody responses [31]. In agreement with this possibility, our results revealed a significant correlation between female sex and 21 day anti-SARS-CoV-2 RBD titer, as also reported in the literature in a different context [32].
The relationship between sex and COVID-19 has specific characteristics at various levels. For example, in the pre-vaccination period, female sex has been identified as an independent risk factor for SARS-CoV-2 seropositivity among blood donors in Wuhan, thus suggesting that estrogen receptor signaling may influence the immune response to SARS-CoV-2 infection [33]. In contrast, compared with men, women require shorter hospital stays and have a lower mortality risk [34].
In the present study, we did not find any association between smoking habit and severe adverse events and, accordingly, we observe no different distribution of 21 days anti-SARS-CoV-2 RBD titer between never smokers and current/former smokers participants. Actually, the effect of smoking on final antibody level after immunization has shown varying results with different viral vaccines. For example, a very low antibody response was reported in smokers after the hepatitis B vaccine [35]. Similarly, it was observed that the antibody response in smokers decreased notably after the influenza vaccine [35]. In contrast, different studies reported no correlation between smoking and antibody level after influenza vaccination [25]. Moreover, we did not find any association between systolic and diastolic pressure and post-vaccination adverse events. Although this finding was rather expected because all study participants were not affected by arterial hypertension, however this a significant result because blood pressure levels have been associated with lower Ab titres following COVID-19 vaccination [36] as well as COVID-19 vaccination was reported to affect blood pressure control [37].
The study regression model also indicated that individuals reporting severe adverse effects, such as dyspnea, paresthesia and fever ≥ 39°C after the second dose of the Pfizer-BioNTech BNT162b2 vaccine, had a high anti-SARS-CoV-2 RBD titer on the day of the second dose. Although an ideal COVID-19 vaccine must elicit a high and long-lasting immune response to provide immunity to prevent disease [25, 38], a high neutralizing antibody titer may be a determinant of reactogenicity [39]. This excessive inflammatory response to vaccination may result in the synthesis and abundant release of pyrogenic cytokines (i.e., interleukin-1, IL-6, tumor necrosis factor-α and prostaglandin-E2) into the bloodstream, thus mimicking a strong immune response to a natural infection through several pathways such as phagocytosis, complement activation and cellular recruitment. These events may also lead to the development of signs and symptoms of injection-site inflammation, and cause other systemic adverse effects (such as fever and dyspnea).
The prevalence of Vit D insufficiency is high worldwide, particularly among hospital workers [40]. Moreover, the role of vitamin D status in the development of COVID-19 has been widely analyzed, and abundant evidence has been reported. Indeed, severe Vit D deficiency increases the probability of death by 50% in patients with COVID-19 [41]. Vit D is an important immune regulator that influences the number of circulating CD4+ and CD8+ T lymphocytes, and its supplementation increases CD4 lymphocytes and regulatory T cell activity [38]. High circulating levels of Vit D in study participants was associated with reported severe symptoms after the second dose of the BNT162b2 vaccine. Although this observation in regression model was not statistically significant, it warrants attention, because participants with severe symptoms had higher anti-SARS-CoV-2 RBD titers.
Our work has clear limitations. First, our study mainly used a time limited exploratory observational approach, and no pathophysiologic mechanisms were assessed. A second limitation of this study was the relatively low number of participants. Moreover, the questionnaire used to collect local or systemic adverse effects reported after the second dose of the BNT162b2 vaccine is not validated, although it was routinely administered to HCWs undergoing COVID-19 vaccination as the COVID-19 vaccination campaign began in our hospital. Further research is needed to verify the role of anti-SARS-CoV-2 RBD titer in the intensity of systemic reactions leading to severe adverse effects after COVID-19 vaccination. Finally, we did not collect blood samples for further analysis after the second dose of the BNT162b2 vaccine. This is another important limitation of the study design because to test again the anti-SARS-CoV-2 RBD titer could have strengthened the study results. Moreover, the response rate to the invitation to participate in this observational study was quite low (28%). However, we did not observe any significant differences between responders and not-responders to the survey in terms of age, sex or professional role. Possible explanations are that, although the importance of the research in educating the community about the vaccine’s adverse effects, we did not use any hospital advertising posters or contacts by mail, and participants were not given any incentives to participate in the study. In conclusion, our study underscores that mRNA vaccines require continual monitoring with spontaneous or active surveillance systems, and a subsequent thorough evaluation of the reports by national institutions responsible for pharmacovigilance. Specifically, our study confirms that women have a markedly higher risk than men of developing severe adverse effects after the second dose of the Pfizer-BioNTech vaccine. Moreover, as reported in very recent publications [39, 42, 43], documenting a possible link between the intensity of symptoms and the anthropometric and biochemical characteristics of survey participants, we believe that our results reinforce the need of a line of research that may help prevent COVID-19 vaccine hesitancy.
CONCLUSION
In our cohort of healthcare workers, we found that the frequency of adverse events tended to higher after second dose than after first dose of the BNT162b2 vaccine, in particular fatigue, headache and fever ≥ 39°. High anti-SARS-CoV-2 RBD titer on the day of the second dose and female sex are the main determinants of the development of adverse events after the second dose of the BNT162b2 vaccine. Therefore, we think that our main findings reinforce the need of a line of research that may help prevent COVID-19 vaccine hesitancy. Moreover, more research on the relationship between adverse effects and the number and timing of repeated vaccinations is required.
AUTHORS’ CONTRIBUTION
Conceptualization, L.C. and S.R.; methods, L.C. and S.R.; validation, S.R. and A. Magrini; formal analysis, L.C. and S.R.; investigation, G.F., A.N., S.L., C.F. and A. Mazza; resources, S.R. A. Magrini; data curation, G.F., A.N., C.F. and A. Mazza; writing—original draft preparation, G.F., A.N. and S.L.; writing—review and editing, L.C. and S.R.; visualization, A. Mazza; supervision, A. Magrini; project administration, L.C. and S.R. All authors have read and agreed to the published version of the manuscript.
KEY MESSAGES
• Although COVID-19 vaccines have clearly contributed to the substantial decreases in COVID-19 mortality the major determinant of COVID-19 vaccine hesitancy is a fear of adverse effects associated with COVID-19 vaccines.
• We observed that most study participants (83%) (206 hospital employees undergoing BNT162b2 mRNA vaccination) reported no symptoms or mild to moderate symptoms after the first dose of BNT162b2 vaccination whereas fatigue headache and fever ≥ 39°C tended to be higher after the second dose.
• We found that the female sex and the high anti-SARS-CoV-2 receptor-binding domain titer after the first dose increases the risk of vaccine-related systemic adverse after the second dose of the Pfizer-BioNTech vaccine among HCWs.
• Our study underscores the need for more research on vaccination side effects and in particular studies that investigate the relationship between adverse effects and the number and timing of repeated vaccinations (boosters).
LIST OF ABBREVIATION
| HCW | = Healthcare worker |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the Ethics Committee of Polyclinic Tor Vergata, Rome (198/2021).
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committee and with the 1975 Declaration of Helsinki, as revised in 2013.
CONSENT FOR PUBLICATION
Written informed consent was obtained from all participants involved in the study.
AVAILABILITY OF DATA AND MATERIALS
The data and supportive information are available within the article.
FUNDING
None.
CONFLICT OF INTEREST
Dr. Luca coppeta is the Editorial Advisory Board members of the journal The Open Public Health Journal.
ACKNOWLEDGEMENTS
Declared none.


