All published articles of this journal are available on ScienceDirect.
Distribution of Voltage-gated Sodium Channel Mutations in Aedes Aegypti Populations from Rural Areas of Indonesia
Abstract
Introduction:
Semarang has been one of the endemic districts; since 2017, it has contributed to high dengue cases in Central Java. The study was conducted to see the mutation in the Voltage-Gated Sodium Channel (VGSC) Aedes aegypti genera.
Materials and Methods:
Aedes aegypti from 6 sub-districts in Semarang were examined, where each sub-district was taken from 2-3 villages endemic areas with high fogging intensity in Semarang. Ten larvae samples were taken from each village.
Results and Discussion:
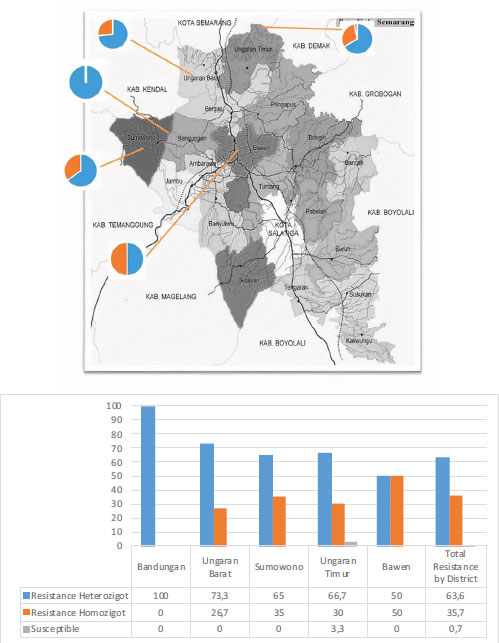
The results showed that the resistant samples were 48.4% heterozygous and 51.6% homozygous resistant from Bawen, 100% heterozygous resistant from Bandungan, 73.3% heterozygous and 26.7% homozygous resistant from West Ungaran, 65% heterozygous and 35% homozygous resistant from Sumowono, 23.3% heterozygous and 66.7% homozygous resistant, and 10% still susceptible from Ambarawa, and 30% heterozygous and 70% homozygous resistant from East Ungaran districts.
Conclusion:
Based on the results of the molecular test, the Ae. Aegypti in Semarang Regency has proven resistance to the pyrethroid insecticide cypermethrin.
1. INTRODUCTION
Dengue Hemorrhagic Fever (DHF) is a disease caused by the Dengue virus. Dengue virus is an Arthropod-Borne Virus, genus Flavivirus, and family Flaviviridae. Dengue Hemorrhagic Fever (DHF) is a disease caused by the Dengue virus. Dengue virus is an Arthropod-Borne Virus, genus Flavivirus, and family Flaviviridae. DHF is transmitted through mosquito bites from the genus Aedes, especially Aedes aegypti or Aedes albopictus. Although it can occur at any time of the year, dengue fever often starts at the beginning of the rainy season. People <15 years of age are more at risk of dengue disease, even though that disease can attack all age groups. The emergence of that disease is closely related to people's behavior, as well as environmental conditions [1]. Epidemiologically, dengue is one of the health problems in the world. Based on WHO data in 2014, 198 million cases of dengue occurred worldwide, and in 2013 there were 584,000 deaths due to dengue. In 2015, according to WHO’s estimates there were 214 million new cases of dengue worldwide, with the death of around 438,000 people [2].
DHF is becoming an increasingly significant health concern for the people of Indonesia. DHF spreads throughout Indonesia; in some areas, it has a high level of endemicity. In 2017, there were 68,407 cases of dengue fever, with 493 deaths. This number decreased quite drastically from the previous year, i.e. 204,171 cases and as many deaths as many as 1,598 people. The DHF morbidity rate in 2017 decreased compared to 2016, from 78.85 to 26.10 per 100,000 population. One of the indicators used to control dengue fever is the Larva Free Rate (ABJ), but until 2017, ABJ nationally had not reached the program target (≥95%); even in 2017, it was only 46.7% [3]. This figure has decreased considerably compared to ABJ in 2016 of 67.6%. In Central Java, all 35 districts/cities have contracted dengue fever. The Dengue Haemoragic Fever (DHF) morbidity (IR) rate in Central Java Province in 2017 was 21.68 per 100,000 inhabitants, a decrease compared to 2016, which was 43.4 per 100,000 population. The Incidence Rate (IR) of DHF in 2017 was 24.3 per 100,000 population of 246 cases found and handled. Crude Fatality Rate (CFR) in 2017 was 0.4% (2 cases). The IR of DHF of Semarang District in 2018 was 16.63 per 100,000 population, while the CFR was 1.16% [1].
One of the efforts to control DHF is chemical control of DHF Vectors, for example, by applying insecticides to fogging. Repeated insecticide application in an ecosystem leads to vector resistance to the insecticides used [4]. Several studies have reported the existence of resistance to various insecticides in mosquitoes. Jahan & Shahid reported that the Lahore, Pakistan population of Ae. Aegypti has been resistant to several studies that have reported the existence of resistance to various insecticides in mosquitoes [5]. In the northeast region of India, Culex quinquefasciatus has been resistant to deltamethrin. Lima's research results state that Ae. Aegypti in Brazil has been resistant to cypermethrin since 2004, 2005, and 2011 [6]. According to Ahmad's research, the Ae. Aegypti caught in Bandung, Surabaya, Jakarta, and Palu were resistant to permethrin and deltamethrin, while mosquitoes caught in Palembang were already resistant to malathion. In Indonesian society, cypermethrin is commonly used to control termites, wood-destroying insects, mosquitoes, flies, roaches, and cockroaches. Research in Yogyakarta reports that Ae. Aegypti was resistant to cypermethrin at 0.05% with an average mortality rate of 4.03% [4].
Cypermethrin insecticide is included in the synthetic pyrethroid (SP) insecticide, which works to disrupt the nervous system causing neurotoxicity. The way cypermethrin works is by disrupting signal transduction in the nervous system and influencing ion transport across cell membranes [7]. Cypermethrin can cause knockdown in insects and works by blocking sodium ion channels found in insect nerve membranes. Fogging is carried out in Central Java using the insecticide malathion and cynof. The pyrethroid insecticide that is the oldest (more than ten years) and often used in Central Java is cypermethrin. Based on data from the Health Office in Semarang, the Health Office in Semarang has used the insecticide cypermethrin since 2013 until now.
The detection of the level of vector resistance to insecticides is used to consider insecticide selection in vector control. The vector resistance to insecticides can be detected by impregnated paper, biochemical or enzymatic detection using microplates, and molecular detection. The basic principle of molecular resistance detection is carried out by genetically identifying vectors against certain insecticide groups used conventionally, one of which is the voltage-gated sodium channel (VGSC) gene. An indicator that the insect vector is resistant to the insecticide pyrethroid group and DDT is the presence of mutations in the VGSC gene. Molecularly, the VGSC gene changes one nucleotide base in the amino acid leucine to phenylalanine which is associated with resistance. The Polymerase Chain Reaction (PCR) method is used for multiplying DNA to detect and determine virus serotypes. The PCR method in this study was used to detect DNA mutations of the VGSC gene as an indicator of resistance.
2. MATERIALS AND METHODS
Semarang is one of the Regency in Central Java Province, Indonesia. The geographic location of Semarang is at 110° 14'54.75 “to 110° 39'3” East Longitude and 7° 3'57 “to 7° 30” South Latitude. The area of Semarang Regency is 95,020,674 Ha. The air temperature in Semarang Regency is relatively cold, around 21.3°C - 31°C. It is because the Semarang Regency is at an altitude of 318 meters above sea level to 1,450 asl.
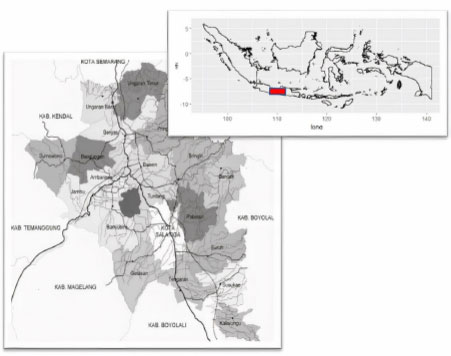
The research was conducted using a pure experimental design. Sampling was conducted in 5 sub-districts in Semarang Regency, namely Bandungan District, West Ungaran District, Sumowono District, East Ungaran District, and Bawen District. Each district is taken from 2 or 3 villages—a map of the study area is presented in Fig. (1). The selected villages are sub-districts endemic to DHF, and in the last three years, fogging has been carried out at least once a year. These are Bandungan, Candi, and Kenteng (Bandungan), Candirejo, Genuk, and Nyatnyono villages (West Ungaran districts), Jubelan and Sumowono sub-districts (Sumowono sub-districts), Kawengen village, Kalongan district, and the village of Kalirejo (District of east Ungaran), the village of Asinan, the village of Bawen, and the village of Harjosari (District of Bawen).
The research procedure is presented in Fig. (2). The research procedure involved placing ovitraps at various predetermined locations within a village. Ovitrap (abbreviation of oviposition trap) is an egg trap, which is a device for detecting the presence of mosquitoes. In particular, the ovitrap is used to detect mosquito infestation in new areas where mosquitoes have yet to be studied. The ovitrap model can be seen in Fig. (3).
After seven days, the ovitrap was taken, and the eggs were hatched in the laboratory. After the eggs hatched and the mosquitoes matured, they were placed in a cage for mating and egg-laying. The resulting eggs (F1) were then hatched to become larvae. For the larvae to survive, they were fed with dog food. These larvae were subsequently utilized as research specimens. So, the sample used was Ae. Aegypti larva, which was the result of rearing (F1). F1 larvae were selected to reduce the risk of exposure to other insecticides from the environment. In each village, ten samples were collected using a random sampling technique.
The primary data in this study is the result of the detection of Ae. Aegypti mosquito resistance. The resistance detection test was carried out at the Banjarnegara Research and Development Center using the Polymerase Chain Reaction (PCR) method. The PCR method is one of the methods used to multiply DNA. PCR is important as a diagnostic tool to detect and determine virus serotypes. With this method, the mutation of the gene of VGSC Aedes aegypti at codon 1016 can be known. The data were then analyzed descriptively to describe the research results. The gene fragment was amplified by PCR using a primer Ile1016r/ Val1016f/ Ile1016f/ Gly1016r/ Val1016f/ Gly1016f.
PCR was performed using SYBR green (Toboyo, US), and PCR machine illumina Rv3. The PCR test was conducted by adding 600 ul nuclei lysis solution into a 1.5 ml tube and chilling it. Then, 10-20 mg (±20) larvae were added to the solution and it was homogenized for 10 seconds. About 17.5 µl of K kinase protein was then added to the mixture, and incubated for 15-30 minutes at 65°C. 3 µl RNAase solution was then homogenized and incubated for 30 minutes at 37°C. The solution was subsequently left at room temperature for a duration of 5 minutes. 200 µl of protein precipitation solution was added, and the resulting mixture was mixed by vortexing for 20 seconds. The sample was subsequently placed on ice for 5 minutes and then centrifuged at 13,000 rpm for 4 minutes. Meanwhile, 600 μL of isopropanol was prepared in a new 1.5 mL tube. The supernatant was transferred to a tube containing isopropanol. The solution was gently stirred until white threads formed, and then it was centrifuged at 13,000 rpm for 1 minute at room temperature. After removing and discarding the supernatant, 70% ethanol was added at room temperature and centrifuged at 13,000 rpm for 1 minute at room temperature. The ethanol was obtained using a micropipette and filter paper. The pellets were dried at room temperature for 10-15 minutes. The DNA pellet dissolved with 100 µl of DNA dehydration solution. The sample was then incubated at 65°C for 1 hour (or 40°C for 24 hours). The PCR test consists of an initial denaturation step of 12 minutes at 95°C and a step of 39 cycles of denaturation of 20 seconds at 95°C, annealing of 60 seconds at 60°C, and an elongation of 30 seconds at 72°C, the last stage of an additional extension stage of 5 minutes at 72°C.
The PCR products were electrophoresed on 2% agarose gel Tris/Borate/aminetetraacetic acid (EDTA) buffer (TBE buffer, 89 mM Tris, 89 mM boric acid, 2 mM EDTA). Gels were run for 90 minutes at 80 volts, and evaluated under UV light (300 nm). Samples containing PCR products were at ± 82 bp and 60 bp of base weight, respectively. The master mix consisted of 5 µL DNA template, 24 µL ddH2O, 10 µL Go Taq and 1 µL primer Ile1016r/ Val1016f/ Ile1016f/ Gly1016r/ Val1016f/ Gly1016f each.
Secondary data were obtained from related agencies, namely the Health Office in Semarang Regency and the public health center where the research was conducted, which was used as a reference in carrying out the research.
| Sub-district | Village | Target DNA Results | Interpretation | Number |
|---|---|---|---|---|
| Bandungan | Bandungan | 60 bp & 80 bp | Heterozygous resistant | 10 |
| - | Candi | 60 bp & 80 bp | Heterozygous resistant | 10 |
| - | Kenteng | 60 bp & 80 bp | Heterozygous resistant | 10 |
| West Ungaran | Candirejo | 80 bp | Homozygous resistant | 2 |
| 60 bp & 80 bp | Heterozygous resistant | 8 | ||
| Genuk | 80 bp | Homozygous resistant | 2 | |
| - | - | 60 bp & 80 bp | Heterozygous resistant | 8 |
| - | Nyatnyono | 80 bp | Homozygous resistant | 4 |
| - | - | 60 bp & 80 bp | Heterozygous resistant | 6 |
| Sumowono | Jubelan | 80 bp | Homozygous resistant | 2 |
| - | - | 60 bp & 80 bp | Heterozygous resistant | 8 |
| - | Sumowono | 80 bp | Homozygous resistant | 5 |
| - | - | 60 bp & 80 bp | Heterozygous resistant | 5 |
| East Ungaran | Kawengen | 80 bp | Homozygous resistant | 4 |
| 60 bp & 80 bp | Heterozygous resistant | 6 | ||
| Kalongan | 80 bp | Homozygous resistant | 8 | |
| - | - | 60 bp & 80 bp | Heterozygous resistant | 2 |
| - | Kalirejo | 60 bp | Susceptible | 1 |
| - | - | 80 bp | Homozygous resistant | 8 |
| - | - | 60 bp & 80 bp | Heterozygous resistant | 1 |
| Bawen | Asinan | 80 bp | Homozygous resistant | 6 |
| - | - | 60 bp & 80 bp | Heterozygous resistant | 4 |
| - | Bawen | 80 bp | Homozygous resistant | 6 |
| - | - | 60 bp & 80 bp | Heterozygous resistant | 4 |
| - | Harjosari | 80 bp | Homozygous resistant | 3 |
| - | - | 60 bp & 80 bp | Heterozygous resistant | 7 |
| - | Number of samples | - | - | - |
3. RESULTS AND DISCUSSION
Sample examination was performed using Val-R, Gly-R primers, and V1016 G F, with DNA targets 60bp (susceptible), 80 bp (homozygous resistance), 60 & 80 bp (heterozygous resistance). Test Results of Mosquito Larva Susceptibility Ae. Aegypti to the cypermethrin will be presented in Table (1).
In this study, 14 sub-districts were taken, in which ten larvae samples were taken for each sub-district. From the results of this examination, all samples of Ae. Aegypti in Bandungan District have a heterozygous resistance status. Of the 140 samples examined, only one sample is still susceptible, namely the sample from the sub-district of Kalirejo, East Ungaran District. Overall, of the 140 samples, 89 samples (63.6%) showed heterozygous resistance, 50 samples (35.7%) showed homozygous resistance, and only 1 sample (0.7%) larvae Ae. Aegypti, which is still susceptible to the insecticide cypermethrin. Fig. (4) displays the percentage of cypermethrin resistance by sub-district area.
The studies on insecticide resistance aim to provide crucial information regarding the mechanisms of resistance. This study examined a mutation in the VGSC gene, where pyrethroid insecticides cause a mutation of this type. This VGSC gene mutation occurs not only in Indonesia but also in several regions of the world. On the American continent, mutations in the VGSC gene in Ae. Aegypti population was first reported in Panama. In Murcia's report, VGSC mutations occur in codons V1016G and I1011M [8]. In Taiwan, research on the Ae. Aegypti detected that 28.3% of the samples had mutations at codon V1016G, 17.83% of samples had mutations in codon S989P, 21.97% of samples had mutations in codon F1534C, and 66.69% of samples had mutations at codon D1763Y [9]. In Indonesia, research on mutations in Ae. Aegypti samples came from Palembang, where in this study showed the results of the V1016G gene with a DNA target of 82 bp. In Padang, mutations were found in VGSC codons V1016G and S989P of Ae. Aegypti with a DNA target of 579 bp [10]. Research in West Sumatra found mutation points in the VGSC gene, where positive results were found in codons S989P and V1016G [11]. In Palu, mutations in the VGSC codon were detected in samples examined from Balaroa, where the codon V1016G and S989P were targeted at 619 bp DNA [12].
The results of this study indicate that the Ae. Aegypti larvae examined have undergone mutations and selective suppression of the insecticides from the pyrethroid group (in this case, Cypermethrin). It is released because the insecticides from the pyrethroid group are widely used in the community, for example, as household insecticides. This causes Ae. Aegypti to be frequently exposed to insecticides from this group and is exacerbated by using these insecticides in the health sector. The Health Office in Central Java Province informed that several cities in Central Java Province have used cynof insecticide in addition to Malathion for fogging to control Ae. Aegypti. In the V1016G mutation, what occurs is a change in the codon coding for valine to glycine, where the base transition of thymine and guanine occurs in the GTA to GGA [13]. The results of this study showed that most of the Ae. aegypti in Semarang Regency has undergone a V1016G mutation in the VGSC gene which is the target of pyrethroid synthetic insecticides.
The occurrence of Ae. Aegypti against pyrethroid insecticides can be detected molecularly; for example, a gene mutation occurs with a target mutation in the VGSC gene for pyrethroids, which can cause conformational changes in sodium channels. This is because these sodium channels cannot be opened by insecticide molecules [14]. Detection of the presence of VGSC gene mutations directly can be done by assessing the transformation of target cells, becoming the insecticide target. The basic principle of molecular detection of vectors' resistance is to identify their genes. The way pyrethroid insecticides work is by attaching to the VGSC section, which is in the vector insect neurons [15]. The mechanism of action of pyrethroid insecticides is by binding to the VGSC protein, which regulates the insect nerve impulses. The pyrethroid insecticide molecules will initially attach to the insect neurons to open sodium channels and then bind them to remain open. This situation will lead to repetitive energizing, which will cause the movement or activity of insects to become uncontrolled. In normal insects that experience repetitive energy, this can cause their flying behavior to become uncontrollable due to convulsions [16]. However, in insects that have mutations in the VSGC gene, the amino acids produced will change. This has an impact on reducing the sensitivity of pyrethroid insecticide molecules to form bonds in these genes.
Pyrethroid insecticide resistance is detected by the molecular test in two ways: detecting changes in detoxification enzymes or changes in the target VGSC site. Detection of changes in detoxification enzymes is to detect gene point mutations that can cause increased levels of enzymes that function to detoxify insecticides, often referred to as metabolic resistance [17]. In this study, researchers only tested molecularly by looking at changes or mutations in the VGSC gene. The expression of specific genes and autosomes is thought to affect the locus of genes, especially if there is a resistance effect from exposure to insecticides. Gene mutations in mosquitoes Ae. Aegypti is important to study because the Ae. Aegypti is the major vector of the dengue virus, so this makes it difficult to control DHF. Insecticide resistance causes the mosquito population to remain high even though insecticide has been applied. So the mosquito vector of dengue fever is difficult to eliminate. Mutations in the VGSC gene can also be an indicator of the development of resistance of Ae. Aegypti mosquitoes to certain insecticides [18]. This is very important because it can be used for evaluation and improvement in efforts to prevent disease prevention. For example, research on genetic mutations in species Ae. Aegypti, where these species have experienced selective suppression of insecticides from the pyrethroid group [19]. As we know, the pyrethroid group of insecticides is widely used by the public or households, causing the Ae. Aegypti mosquito is often exposed to insecticides from that class and can be exacerbated by exposure to insecticides from other groups. Based on the results of this study, it was found that from the susceptibility test, the Ae. Aegypti in Semarang Regency has been resistant to the insecticide Cypermethrin. This allows for another resistance mechanism (molecular mechanism) that also takes place in these mosquito species. This resistance is due to using pyrethroid insecticides for a long time and is carried out continuously. Initially, pyrethroid insecticides can kill almost 100% of Ae. Aegypti mosquitoes. Still, in a certain period, insects will remain alive, which at first are only a few, but then the numbers increase. The living mosquito population will continue to reproduce as well as pass on the ability to be resistant to pyrethroid insecticides to future offspring.
The results showed that only 0.7% of the sample was susceptible, and the rest had mutations. In this study, two types of mutations were found in the sample, namely homozygous (G / G) and heterozygous mutations (V / G). This means that from all samples examined, only 0.7% of samples of mosquito larvae had not undergone mutation (V / V), and 35.7% of samples of mosquito larvae had homozygous mutations (G / G) in the 1016G and 63 alleles. 6% of mosquito larvae samples had heterozygous mutations in the second domain of the V1016G gene. The high case of resistance follows the research conducted in Yogyakarta, where the Ae. aegypti that has been obtained to exhibit resistance to pyrethroids. Ae. aegypti mosquito that experienced homozygous resistance was almost the same as the Ae. aegypti with heterozygous resistance. However, this study has not found any generation with strains susceptible to pure homozygous. Resistance can be genetically inherited, and the level of resistance development is influenced by the frequency of the resistance gene, the frequency of exposure to the insecticide, and the duration of insecticide application. The results of molecular screening on genes for Ae. Aegypti found target-site mutations and high kdr V10161 mutations (87%). The process of forming a susceptible homozygous strain takes a long time, namely over five generations [20]. A research result shows that only 0.75% of individual Ae. Aegypti with homozygous V1016G mutations were able to survive or were resistant to exposure to the insecticide permethrin, whereas those with heterozygous mutations and were able to survive were only 23.4% [21]. It shows that the resistance mechanism to pyrethroid insecticides in Ae. Aegypti mosquitoes are influenced by several factors, for example, environmental factors. The resistance of Ae. Aegypti to synthetic insecticide pyrethroids in the VGSC gene is not only at the V1016G position, but also in other positions, for example, F1534C, and S989P [22].
There are 1 out of 140 samples of mosquito larvae that have not mutated; this is far below the results of research in Thailand in 2013, which showed that 74 out of 170 samples of Ae. Aegypti mosquito larvae (43.5%) were still susceptible or had not mutated (V / V) [23]. Ae. aegypti, which is still susceptible, was also found in West Bengal, India, where the results of the examination on five samples showed no mutations in the 1016G allele, so it can be said that no resistance was found; both homozygous and heterozygous mutations, which means that all samples are still susceptible. In the area where the Ae. Aegypti is still susceptible; pyrethroid insecticides must be regularly monitored and regulated to prevent resistance. Polymorphisms in target genes should also be monitored regularly to detect the early emergence of pyrethroid resistance in the population of Ae. Aegypti [24].
Based on the study's results, it is known that 63.6% of the Ae. Aegypti samples examined had heterozygous mutations. The 1016G allele is recessive; this proves that the Ae. Aegypti mosquito with a heterozygous mutation is still likely to remain sensitive to pyrethroid insecticides [23]. The incidence of heterozygous resistance rarely occurs in a population. Still, if a heterozygous mutation appears and can survive until mating with other heterozygotes, it will produce homozygous mutants with stronger insecticide resistance properties. If these homozygous mutants are dominant in a population, resistance will spread rapidly in the population because the Ae. Aegypti mosquito will easily adapt to its environment [11].
Insect resistance to an insecticide can occur if the insecticide is used continuously for 2-120 years. Cypermethrin, alfa cypermethrin, deltamethrin, and lambda cyalothrin are active insecticide ingredients from the synthetic pyrethroid group of type II. Cypermethrin is one of the active ingredients most often used in mosquito vector control programs. Examples of insecticide active ingredients, synthetic pyrethroids of type I, are transfluthrin, prallethrin, esbiotrhrin, d-allethrin, and meperfluthrin. These active ingredients are often used in household insecticides [14]. The chemical composition of the synthetic pyrethroid groups of type I and II is almost the same; it is just that the synthetic pyrethroid group of type II has a cyano (CN) group, while the synthetic pyrethroid group of type I does not have this group. The function of this cyano group is to increase insecticidal activity. This causes the synthetic pyrethroid type II group to have a higher insect killing power of up to 3-6 times than the synthetic pyrethroid of the type I group and also triggers resistance to other synthetic pyrethroid groups [5].
Although most of the samples (63.6%) showed heterozygous mutations, determining the type of insecticide to be used in the control program of Ae. Aegypti mosquito (in the form of fogging) must consider the possibility of resistance. The occurrence of mutations against one type of insecticide can cause insect resistance to other insecticides in a group [25]. In this case, the mutation of cypermethrin can lead to mutations of other insecticides in the synthetic pyrethroid class. The variation in resistance levels in each strain can be caused by differences in the level of use and types of insecticides in each region, genetic background, and ecological variations. Therefore, the insecticide application should not be used continuously but given a pause to give the susceptible genotype a chance to survive. The susceptible genotype can be derived from the inheritance of a heterozygous resistant recessive gene resulting from crossbreeding between susceptible and resistant individuals. Suppose the number of individuals with heterozygous mutations is higher than those with homozygous mutations. In that case, this can accelerate the occurrence of resistance in the future because there is no protective mechanism in mosquitoes. This protection mechanism in mosquitoes depends on genetic factors (single or recessive, semi-dominant or dominant).
CONCLUSION
Based on the results of the molecular test, the Ae. Aegypti in Semarang Regency has proven resistance to the pyrethroid insecticide cypermethrin. Whereas until now, DHF control by using the fogging method using the pyrethroid insecticide cypermethrin is still being carried out in Semarang Regency. The molecular test in this study showed a mutation in the VGSC gene codon 1016 in the Ae. Aegypti, which means the Ae. Aegypti is already resistant to the pyrethroid insecticide cypermethrin.
LIST OF ABBREVIATIONS
| VGSC | = Voltage-Gated Sodium Channel |
| DHF | = Dengue Hemorrhagic Fever |
| IR | = Incidence Rate |
| CFR | = Crude Fatality Rate |
| SP | = Synthetic Pyrethroid |
| PCR | = Polymerase Chain Reaction |
ETHICAL STATEMENT
The study was approved by the Health Research Ethics Commission, Universitas Negeri Semarang. Letter Number: 168/KEPK/EC/2019.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
This research was funded by PNBP funds from the Universitas Negeri Semarang.
CONFLICT OF INTEREST
The authors declare no conflicts of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The researcher would like to thank the Semarang State University for funding this research. Thanks, are also given to the entire research team involved in this study.


