All published articles of this journal are available on ScienceDirect.
Immunostimulant and Pharmacological Activities, of Cucurbita Maxima Seeds on Humoral and Cellular Immunity Cells and their Functions
Abstract
Background
Natural resources are key for discovering medicinal compounds. Hence, the objective of this study was to evaluate the immunomodulatory activities of Cucurbita maxima (pumpkin) seeds, a component of Moroccan folk medicine believed to enhance immunity.
Methods
We produced three distinct extracts employing ethanol, ethyl acetate, and water as solvents. The immunomodulatory effects of these extracts were assessed on rabbit immune cell proliferation and their functions, including IgG production, cytotoxicity, and phagocytosis.
Results
The study findings reveal a significant stimulation in thymocyte proliferation, yielding a remarkable 285.7% response, along with a 146.5% increase in cytotoxicity (MLR) in response to the aqueous extract. Furthermore, there was a notable enhancement in complement activity by 119.9% in response to the aqueous extract, implying the potential of C. maxima to modulate both humoral and cellular immunity. Additionally, both EtOAc and aqueous extracts led to a reduction in macrophage phagocytic function. The assessment of the antibacterial properties of ethanol extract showed greater effectiveness against Bacillus, E. Coli, and Staphylococcus. In a separate analysis, antioxidant activity was gauged through DPPH, FRAP, and ABTS methods. These tests exhibited robust antioxidative effects across all extracts, characterized by higher levels of phenolic and flavonoid content. The FT-IR spectrum indicated the presence of compounds such as triglycerides, sterol esters, phospholipids, and unsaturated fatty acids in the extracts.
Conclusion
Collectively, these outcomes prompt the consideration of utilizing the aqueous extract of Cucurbita maxima to strengthen humoral and cellular immunity, as well as lymphocyte toxicity, in various ailments.
1. INTRODUCTION
Immunomodulation represents a therapeutic strategy directed at the targeted adjustment of innate or adaptive immune system components using pharmacological agents or other substances, aiming to achieve either positive (immunostimulatory) or negative (immunosuppressive) effects. Consequently, compounds of either natural or synthetic origin that alter the immune response, whether generally or specifically through stimulatory or suppressive actions, are categorized as immunomodulatory drugs [1].
Moroccan culture is replete with examples of the utilization of pharmacologically active natural products [2], many of which were employed as immunomodulators. Furthermore, the emergence of oxidative stress triggered by free radicals or reactive oxygen species has been linked to severe health implications. This underscores the importance of investigating the biological impacts of medicinal plant extracts to unearth novel, safe, and potent bioactive compounds possessing immunomodulatory, antibacterial, and antioxidant properties.
Cucurbita Maxima, commonly known as pumpkin, has been recognized as a functional food, ranking among the most popular vegetables globally. Its seeds are abundant sources of bioactive agents boasting nutraceutical attributes. Pumpkin seeds are characterized by high contents of protein, fiber, and fat, reaching percentages of 34.56%, 2.91%, and 36.70%, respectively, alongside relatively lower proportions of sugars and starch, accounting only for 1.08% and 2.15%, respectively [3-5]. Notably, these seeds have numerous biologically active substances that exert substantial pharmacological effects, including antioxidant [6], anti-inflammatory, analgesic [7], and hepatoprotective properties [8].
While prior investigations have delved into the chemical composition, antioxidant capacity, and antibacterial properties of different pumpkin varieties worldwide [3-6], studies focused on the biological characteristics of Moroccan cultivars remain scarce. With this in mind, the present study was conceived to explore not only the immunomodulatory influence of Cucurbita seeds on a cell culture model of immune cells through the evaluation of immune function (such as antibody response, T-lymphocyte toxicity, and the complement system) but also to scrutinize the in vitro antioxidant potential, look into the functionality of active components via FT-IR spectroscopy, and investigate the antibacterial aspect. These efforts are geared towards uncovering potential applications in the realms of both food and pharmaceutical industries.
2. MATERIALS AND METHODS
2.1. Preparation of Extracts
Seeds of C. maxima (pumpkin) were procured from a local market, washed twice with distilled water, and then desiccated at 40°C until a consistent weight was attained. Subsequently, they were finely ground for the purpose of defatting. The defatted powder underwent extraction via maceration, utilizing water, ethanol (EtOH), or ethyl acetate (EtOAc) at a concentration of 10%. After filtration, the water extract underwent a 24-hour process of lyophilization. The ethanol and ethyl acetate extracts were evaporated using a rotary evaporator. Finally, all extracts were preserved at -20°C until their intended use.
2.2. Cell Culture
Cell suspensions employed in this study were derived from rabbits that were humanely euthanized. Rabbits weighing between 1.5 to 2 kg were euthanized under petroleum-ether anesthesia. All experimental procedures adhered to ethical regulations and followed animal care guidelines stipulated by the local Ethics Committee CEFST and approved under reference number 13 /2021/CEFST.
The immune organs, namely the spleen and thymus, were aseptically excised from the animals. Cell suspensions were prepared by gently pressing the organs through a fine wire mesh, following the methodology outlined in our previous research [9-12]. The cells were subsequently washed with RPMI medium, and red blood cells were lysed using 154 mM ammonium chloride. The viability of cells was assessed through microscopic examination employing a 0.1% trypan blue exclusion test.
The culture medium used was RPMI supplemented with 2 mM glutamine, 10% serum, antibiotics (ampicillin 100 U/mL and streptomycin 100 mg/mL), and antifungal agent (Fluconazole 2 mg/mL).
2.3. Cell Proliferation Assay
Cell proliferation was assessed using the MTT assay following the protocol by Mosmann et al., 1983, as previously outlined [11-13]. In brief, cells were seeded at a density of 150,000 cells per well in 96-well plates and subsequently incubated at 37°C within a humidified chamber under an atmosphere consisting of 95% air and 5% CO2 for a duration of 72 hours. Prior to the incubation, extracts at the desired concentrations were introduced to the cells and diluted in RPMI medium. Following this, a 10 µL volume of MTT solution (5 mg/mL in PBS) was administered. After three hours of incubation, DMSO was introduced to all wells to dissolve the generated formazan. Finally, the spectrophotometer (BioTek L800) was employed to measure the optical density at a wavelength of 570 nm.
2.4. Isolation of Macrophages and Evaluation of their Proliferation and Activity
To outline, macrophages were isolated from spleen cells based on their capacity to adhere to plate wells. To achieve this, 100 µL of a spleen cell suspension at a concentration of 2.106 cells/mL was added to individual wells of a 96-well plate, followed by an incubation period of 2 hours at 37°C to facilitate macrophage adherence. Subsequently, non-adherent cells were removed, and each well was subjected to two washes with sterile RPMI.
For the assessment of macrophage proliferation, adherent macrophages were incubated with various extracts in RPMI. The evaluation of proliferation was conducted using the MTT assay, as previously described.
The phagocytosis assay was conducted according to our prior studies [9, 11-13], employing neutral red. Specifically, adherent macrophages within each well were incubated with 100 µL of RPMI supplemented with 0.075% neutral red. Subsequently, 10 µL of plant extracts were introduced, and the plates were incubated for 2 hours. Ultimately, after discarding the supernatant and rinsing the cells, the reaction was terminated by employing a solution composed of acetic acid (1M) and ethanol (1:1 v/v), which effectively dissolved the phagocytosed neutral red. The degree of phagocytic activity was quantified by measuring the absorbance at 540 nm, corresponding to the peak absorption of the utilized neutral red in the assay.
2.5. Allogenic Mixed Lymphocyte Reaction (MLR)
Freshly isolated thymocytes obtained from the thymus were subjected to incubation with Chicken Red Blood Cells (CRBC) at a ratio of 105 CRBC to 106 thymocytes in RPMI medium supplemented with serum. This mixture was then incubated for 24 hours at 37°C in a humidified environment, with the extracts either present or absent. The cytotoxicity of thymocytes against CRBC was evaluated by detecting the lysis of CRBC in the medium through measurement of absorbance at 540 nm.
2.6. Complement Test
The complement test was conducted through the assessment of lysis of mouse red blood cells (MRBC) by means of the complement pathway, aided by the presence of antibodies against MRBC. These antibodies were generated by immunizing rabbits using Freund's adjuvant and utilizing mouse RBC as antigens. MRBC were incubated in RPMI medium supplemented with the serum that contained specific anti-MRBC antibodies for a duration of 4 hours, while extracts at a concentration of 2 mg/ml were present, and the incubation was maintained at 37°C. Following the incubation period, the samples were subjected to centrifugation, and the absorbance of the supernatants was measured at 540 nm.
2.7. Evaluation of Total IgG Production by ELISA Assay
The ELISA assay was conducted following the previously established procedures [12, 14-16]. Isolated splenocytes were cultured in RPMI with or without extracts for a duration of 72 hours at 37°C. Subsequently, 100 µL of cell culture supernatant was dispensed onto a microtiter plate for the purpose of IgG assessment. Subsequent to this, a peroxidase-labeled anti-IgG rabbit antibody was introduced (100 µL per well) and incubated for two hours at 37°C. The immune complex was revealed through the addition of the chromogen orthophenylenediamine (OPD), at a concentration of 0.5 mg/mL. The reaction was halted using 3 M HCl, and the absorbance was measured at 490 nm.
For the specific IgG assay, the splenocyte culture was initiated by adding Ovalbumin to RPMI, both with and without extracts. The assay commenced by coating the wells with 100 µL of Ovalbumin (1 g/mL). Following this, 100 µL of the cell culture supernatant was introduced, and the assay was executed in the same manner as outlined for Total IgG.
2.8. Antimicrobial Activity (Agar Well Diffusion Method)
Cucurbita seed extracts were evaluated against a panel of four Gram-positive bacteria, Bacillus subtilis ILP1428B and Staphylococcus aureus CIP543154 (Pasteur Institute Collection), as well as five Gram-negative bacteria: Pseudomonas aeruginosa ATCC27653 and four strains of Escherichia coli CIP5412 (from the American Type Culture Collection).
The surface of agar plates was inoculated by evenly spreading 100 µl of the microbial inoculum across the entire agar surface. Subsequently, an aseptic perforation of 6 to 8 mm diameter was made using a sterile probe or nozzle, and 50 µl of the samples were introduced into the well. The agar plates were then incubated at 37°C for an additional 24 hours. The diameter of inhibition zones was calculated as previously described [17, 18].
2.9. Total Phenolic Content
The total phenolic content of the extract was quantified using the Folin-Ciocalteu method. This technique involved the blending of 1.5 ml of Folin-Ciocalteu reagent (10%) with 200 µl of extracts previously dissolved in methanol. The resulting mixture was gently stirred and allowed to react for 5 minutes in darkness, followed by the addition of 1.5 ml of sodium carbonate solution (5%). Following a 2-hour incubation period at room temperature and in the absence of light, the measurements were taken at 750 nm. The concentration of phenolic content was determined based on a calibration curve utilizing Gallic acid. The outcomes were presented as milligrams equivalent of Gallic acid per gram of extract.
2.10. Total Flavonoid Content
The quantification of flavonoids was conducted using the Aluminum trichloride (AlCl3) method as described by Bahorun et al., 1996 [19-35]. In this procedure, precisely 0.5 ml of each extract (dissolved in methanol) was combined with 0.1 ml of aluminum chloride (10%), 0.1 ml of potassium acetate (1 M), and 4.3 ml of distilled water. Following shaking and an incubation period of 30 minutes at room temperature, absorbance readings were taken at 415 nm. The concentration of flavonoids was determined through reference to a calibration curve established using quercetin. The results were expressed as micrograms of quercetin equivalent per milligram of extract.
2.11. Total Antioxidant Activity
The DPPH radical scavenging assay was performed following the previously established procedure with slight modifications [10, 19, 20]. In essence, the extracts were diluted in methanol and subsequently combined with a DPPH solution. Absorbance readings were taken at 517 nm.
The FRAP assay was executed according to the method outlined by Oyaizu [21]. Each extract was mixed with 2.5 ml of a 0.2 M phosphate buffer solution (pH 6.6) and 2.5 ml of a 1% potassium ferricyanide solution (K3Fe(CN)6). Subsequently, trichloroacetic acid at a concentration of 10% was introduced to halt the reaction. An aliquot (2.5 ml) of the supernatant, diluted with 2.5 ml of distilled water, was combined with 0.5 ml of FeCl3 solution, and the absorbance was measured at 700 nm.
The ABTS assay was carried out as detailed by Re et al. [22]. ABTS was dissolved in distilled water, followed by the addition of potassium persulfate. The resultant mixture was left at room temperature in the dark for a duration of 12 to 16 hours before the introduction of the extracts. Subsequently, 2 ml of the diluted ABTS+ solution was added to 200 µl of each extract and allowed to stand for 1 minute, and the absorbance was determined at 734 nm.
2.12. UV Absorbance
The UV absorbance of the three extracts was assessed in vitro by creating diluted samples with concentrations of 2 mg/ml in ether. Subsequently, absorbance values were ascertained across the wavelength range from 290 nm to 400 nm, which encompasses the UVB and UVA regions. Under identical conditions, we also determined the UV absorbance of two commercial sunscreens to serve as positive controls.
2.13. Infrared Spectral Analysis
Infrared analysis was conducted using the VERTEX 70 - BRUKER infrared spectrophotometer. A small droplet of the purified extract was cautiously positioned onto the surface of a crystal and secured within the path of the infrared beam. The obtained infrared data was subsequently compared to a table of IR frequencies for analysis.
2.14. Statistical Analysis
Each experimental condition was replicated a minimum of three times (n=3). The provided values are presented as the means ± standard error of the mean (SEM). Statistical analyses were performed using the Student's t-test. Significance was determined at a threshold of p<0.05.
3. RESULTS
3.1. Effect of C. maxima Extracts on Humoral Immunity (Fig. 1, Tables 1, 2)
Fig. (1) illustrates the impact of C. maxima extracts obtained through ethanol (EtOH), ethyl acetate (EtOAc), and water on splenocyte proliferation. It is evident that both EtOH and EtOAc extracts significantly inhibited splenocyte proliferation (with inhibitions of 43.2% and 40.5%, respectively, at a concentration of 2 mg/ml). In contrast, the aqueous extract did not alter this proliferation.

EtOH: Ethanol extract; EtOAc: Ethyl Acetate extract; Water: Aqueous extract.
| Cucurbita Extracts | ||
|---|---|---|
| EtOH(N=6) | EtOAc (N=6) | Water (N=6) |
| 50.5 ± 5.0% | 98.9 ± 10.6% | 100.1 ± 6.7% |
Furthermore, we proceeded to assess the total IgG production by splenocytes under the influence of distinct C. maxima extracts. As depicted in Table 1, the outcomes indicate that only the EtOH extract of C. maxima demonstrated inhibition of Total IgG production (Table 1: exhibiting a 49.5% inhibition).
Lastly, the study delved into examining the complement activity under these various extracts. Notably, the aqueous extract exhibited a slight, though statistically non-significant, stimulation of complement activity, while both the EtOH and EtOAc extracts appeared to induce a minor suppression of complement activity (Table 2).
3.2. Effect of C. maxima Extracts on Cellular Immunity (Fig. 2 Table 3)
We conducted an assessment of the extracts’ impact on thymocyte proliferation. As demonstrated in Fig. (2), it is evident that both the EtOH and EtOAc extracts of C. maxima elicited inhibition of thymic cell proliferation (with inhibitions of 48.2% and 41.1%, respectively). Conversely, under aqueous extraction, a significantly pronounced stimulation of thymic cell proliferation was observed, presenting a remarkable 285.7% response (p<0.001; N=5, Fig. 2).
The evaluation of thymic cell cytotoxicity against foreign cells (MLR), as outlined in Table 3, showthat neither the EtOH nor EtOAc extracts induced significant changes in MLR. However, the aqueous extract demonstrated a notably heightened stimulation of cytotoxicity activity (146.5% response compared to control; p<0.005; Table 3).
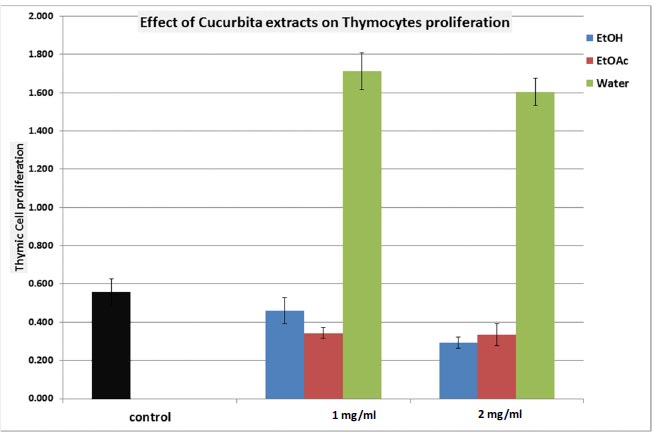
EtOH: Ethanol extract; EtOAc: Ethyl Acetate extract; Water: Aqueous extract.
| Cucurbita Extracts | ||
|---|---|---|
| EtOH(N=6) | EtOAc (N=6) | Water (N=6) |
| 87.9 ± 9.6 | 88.7 ± 9.3 | 119.9 ± 69.2 |
| Cucurbita Extract | ||
|---|---|---|
| EtOH(N=7) | EtOAc (N=7) | Water(N=7) |
| 99.9 ± 17.7 | 110.3 ± 11.6 | 146.5 ± 14.7 |
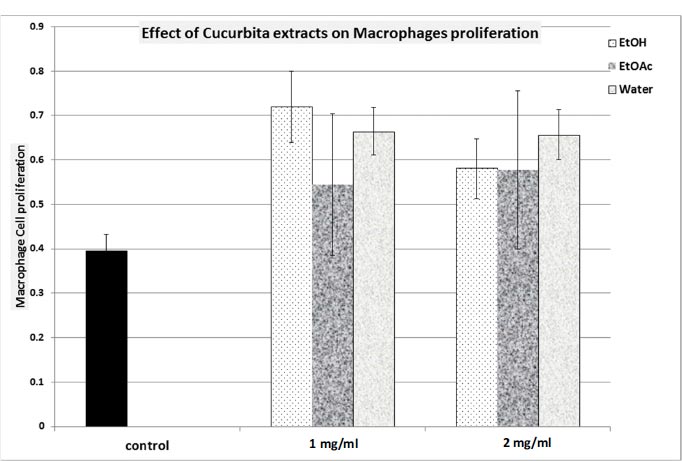
EtOH: Ethanol extract; EtOAc: Ethyl Acetate extract; Water: Aqueous extract.
| N=4 | Control | EtOH | EtOAc | Water |
|---|---|---|---|---|
| Cucurbita | 0.25 ± 0.01 | 0.26 ± 0.04 | 0.15 ± 0.03 | 0.11 ± 0.01 |
3.3. Effect of C. maxima on Macrophage Proliferation and their Activity (Fig. 3 and Table 4)
In terms of macrophage proliferation, we noted that all C. maxima extracts induced marginal stimulation of macrophage proliferation. The highest response was achieved with the EtOH extract, resulting in a maximum response of 187.4% (Fig. 3).
The assessment of phagocytic activity (Table 4) show that the EtOH extract did not bring about any alteration in phagocytosis. In contrast, both the EtOAc and aqueous extracts demonstrated inhibitory effects on phagocytosis (with inhibitions of 40% and 56%, respectively; p<0.05; N=4; Table 4).
3.4. Antimicrobial Activity
The findings pertaining to antimicrobial activity have been consolidated in Table 5. The effectiveness of antimicrobial activity displayed variations based on the specific extract and the targeted microorganism. The aqueous extract emerged as the most potent, exhibiting inhibition potential against Staphylococcus aureus, Pseudomonas aeruginosa ATCC53 (Gram-negative), and E. coli growth. This confirms the immunostimulatory impact of this extract and its potential utilization as a functional food.
3.5. Total Phenolic and Flavonoid Content
Table 6 presents a summary of the outcomes regarding the total phenolic content derived from C. maxima extracts. The observations highlight the higher levels of phenolic and flavonoid content for EtOH and EtOAc extracts compared to the aqueous extracts.
3.6. The Antioxidative Effect of C. Maxima Extracts
To assess the antioxidant activity of C. maxima extracts, three distinct methods were employed: DPPH, FRAP, and ABTS assays.
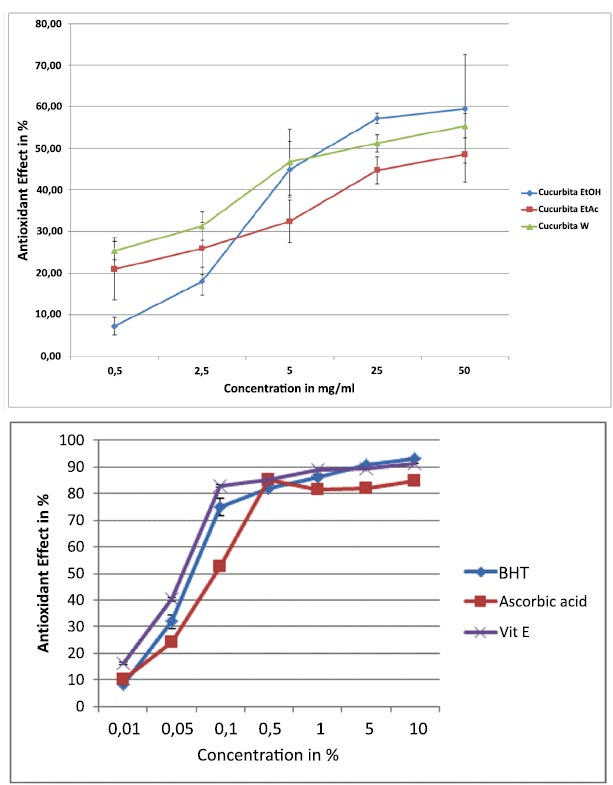
Fig. (4) illustrates the antioxidative impact of C. maxima extracts as determined by the DPPH assay. Notably, all three extracts demonstrated substantial antioxidant effects, which intensified with increasing concentrations ranging from 0.5 to 50 mg/ml. Particularly, the EtOH and water extracts displayed maximal effects exceeding 60%. The IC50 values for EtOH, EtOAc, and water extracts were recorded at 2.5%, 0.52%, and 0.53% respectively. Comparatively, the standard compounds exhibited IC50 values of 0.55%, 0.065%, and 0.085% for Vitamin E, BHT, and ascorbic acid.
Furthermore, the antioxidant power of C. maxima extracts was evaluated through the FRAP assay. As indicated in Fig. (5), the EtOAc extract showcased lower reducing power, while the EtOH and aqueous extracts exhibited robust antioxidant potential at concentrations of 5 and 50 mg/ml.
The antioxidant ABTS assay revealed the potent ability of all the analyzed extracts to effectively quench ABTS+. Notably, the aqueous extract stood out with the highest activity, displaying an IC50 of 7.5 µg/ml (Table 7).
| Bacteria | C. maxima Extracts | ||
|---|---|---|---|
| EtOH | EtOAc | Aqueous | |
| Staphylococcus Aureus Cip (gram+) | + | + | 10 |
| Bacillus subtilis ILP14 (gram+) | - | - | - |
| Bacillus subtilis ILB10 (gram+) | + | - | 8 |
| Pseudomonas aeroginosae ATCC53 (gram-) | 12-11 | - | 10 |
| E.coli DH5α (gram-) | - | - | - |
| E.coli cip 5412 (gram-) | + | + | 10 |
| E.coli AL52 (gram-) | - | - | - |
| E.coli HB101 (gram-) | - | - | - |
| E.coli B12(gram-) | + | - | 8 |
| Samples | Total phenolic content (mg of GA/g Extract) | Total flavonoid content (mg of Q/g Extract) |
|---|---|---|
| EtOH | 686.18± 0.090 | 148.6± 0.070 |
| EtOAc | 790.54±0.0190 | 183.4±0.090 |
| Water | 627.62±0.100 | 92.9±0.041 |
| Samples |
ABTS IC50 (mgTE/edw) |
|---|---|
| EtOH | 9.97± 0.250 |
| EtOAc | 11.45± 0.277 |
| Water | 7.50±0.082 |

EtOH: Ethanol extract; EtOAc: Ethyl Acetate extract; Water: Aqueous extract.
3.7. UV Absorbance
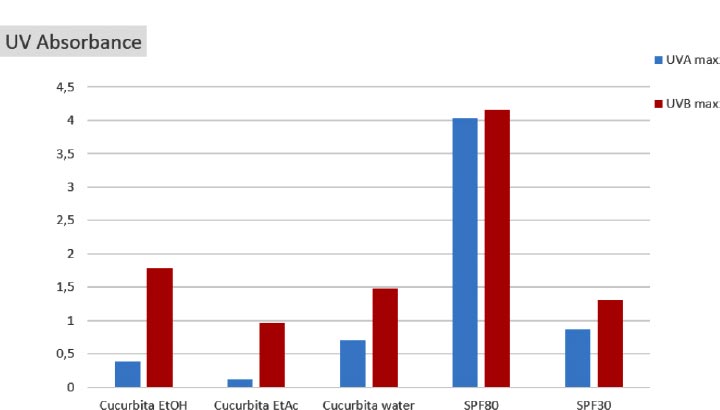
Fig. (6) depicts the peak UV absorbance of C. maxima extracts in comparison to sunscreens SPF80 and SPF30. In the UVB range, it was noted that the EtOH extract exhibited over 1.5 times higher absorption than that of sunscreen SPF30. This was followed by the aqueous extract with 1.5, while the EtOAc extract exhibited the lowest level of UVB absorption, reaching a maximum of 1.
3.8. Infrared Spectral Analysis
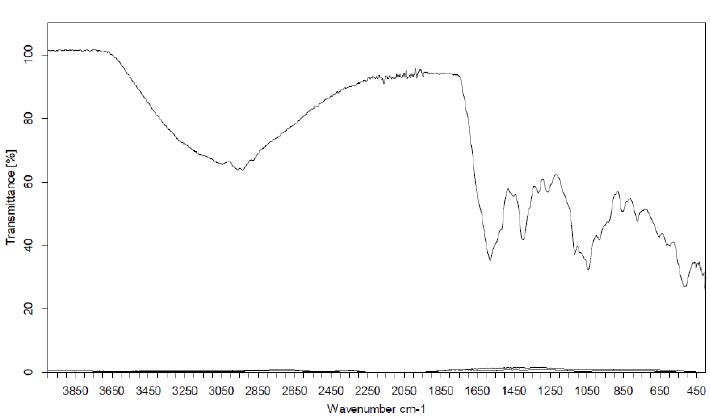
The FTIR spectrum was utilized to identify the functional groups of active components, discerning peak values within the infrared radiation region. The FT-IR spectra of aqueous, ethanolic, and ethyl acetate extracts of C. maxima seeds are presented in Figs. (7 to 9), respectively.
The FT-IR analysis of the aqueous extract unveiled the presence of the aromatic C=C bond observed between 1650 and 1500 cm−1, C—O stretching between 1150 and 1050 cm−1, an out-of-plane O-H bending at 670-640 cm-1, and a robust absorption region between 3500-3000 cm-1 attributable to hydroxyl groups, phenolic/acidic groups, or sugar constituents. These functions boost the extract's polar nature. Peaks between 3000 and 2800 cm-1 correspond to C-H symmetrical stretching. Additionally, an asymmetric CH2/CH3 scissoring at 1420–1470 cm−1 and -CH2 wagging at 880-800 cm−1 were identified. These above-mentioned bands potentially demonstrate the presence of triglycerides, sterol esters, or phospholipids in the extracts [23-25].
Furthermore, distinctive bands situated around 1750–1740 cm−1 (Figs. 8, 9), signifying the stretching of the C=O group, were evident in the organic extracts.
EtOH: Ethanol extract; EtOAc: Ethyl Acetate extract; Water: Aqueous extract.


| Frequency. cm−1 |
Functional Group v: vibration |
Vibration Mode |
|---|---|---|
| 3650-3200 | v(-O-H) (strong) of acidic group, phenolic group and carbohydrate, | stretching |
| 3200-2850 | v(C—H) | stretching |
| 1650-1750 | v(C—H) | stretching |
| 2950-2800 | v(C—H)CH3 + v(C—H) CH2 | stretching |
| 1750 | v(C=O) of carbonyl group of ester (strong and broad) | stretching |
| 1650 | v(C=O) of acidic / amidic group | stretching |
| 1420-1470 | v(C—H) CH2/CH3 | bending (scissoring) |
| 1350-1250 | v(=C—H) | bending |
| 1150 | v(C—O) | stretching |
| 1100-1000 | v(C—H) CH2/CH3 | bending |
| 1000-900 | v(H-C=C—H) | bending |
| 900-450 | v(C—H) CH2 | bending, rocking, wagging |
| 670-640 | v(O—H) | bending |
However, the FT-IR analysis of the EtOAc and EtOH extracts yielded analogous results, both displaying a weak polar nature. Upon spectral examination, an absorption band emerged at 1750 cm−1, indicative of a robust C=O absorption band attributable to the carbonyl ester group [23]. Additionally, the intense peaks at 2850-2860 and 2920–2930 cm−1 corresponded to the symmetric and asymmetric stretches of the CH2 group, while the peak within 2980–3050 cm−1 represented the scissoring vibrations of the CH2 group. All of these characteristics are attributed to unsaturated fatty acids, triglycerides, or unsaturated lipids present in the extracts. This phenomenon was more pronounced in the case of non-polar character extracts such as EtOAc and EtOH than with the aqueous extract [23-25]. The length of the hydrocarbon chains exceeded or were equal to 4 carbon atoms, as evidenced by the presence of a rocking CH2 at 740 cm−1 (Table 8) [26].
4. DISCUSSION
The modulation of immune functions using herbal medicines presents an alternative or complementary approach to conventional therapies for treating infections, immunological disorders, and cancer. Numerous medicinal plants have been evaluated for their immunomodulatory activity in humans and animals, both in vivo and in vitro. The results have consistently demonstrated their beneficial impact on immune system modulation through various mechanisms.
In the Moroccan context, seeds of C. maxima have been utilized in folk medicine to address various ailments. Consequently, the aim of our study was to assess the immunomodulatory effects of three extracts of C. maxima seeds on immune functions.
The EtOH extract of C. maxima inhibited Total IgG production, whereas the aqueous extract remarkably stimulated complement activity, suggesting the potential of C. maxima to modulate humoral immune responses. These findings align with previous research by [27], where C. maxima seed-treated rabbits exhibited increased antibody titers, total white blood cell counts, and serum immunoglobulins in both normal and suppressed animals. Furthermore, C. maxima seeds are renowned for their abundance in bioactive compounds, such as carotenoids, tocopherols, and sterols, which are useful for a broad spectrum of biological activities, as confirmed by in vivo experiments [28, 29].
For cellular immunity, aqueous extracts of C. maxima seeds demonstrated higher stimulation of thymic cell proliferation and Major Histocompatibility Complex (MHC) class II Restricted T-cell proliferation (MLR) activity. This trend is consistent with findings by [27], affirming the potential of C. maxima seeds against cell-mediated immunosuppression in rabbits.
Our observations also revealed that EtOAc and aqueous extracts of C. maxima seeds induced a reduction in the phagocytic function of macrophages. This outcome can be attributed to the anti-inflammatory activity of these extracts, in line with the results obtained by [7]. When evaluating the safety profile and exploring the analgesic and anti-inflammatory activity of ethanol extract of Cucurbita maxima seeds, the seed extract of C. maxima was found to be safe and showed significant analgesic and anti-inflammatory activity in comparison with the control group. The results were similar to the study of [30]. Cucurbita moschata Dush, a plant belonging to the Cucurbitaceae family, was examined in mouse splenocytes and macrophage cell lines. The primary component in Dush is β-carotene. According to the results, both have an immune-enhancing effect by activating macrophages and splenocytes to produce Th1 cytokines. This suggests that β-carotene has an anti-inflammatory effect by significantly upregulating the production of TNFα, IFNγ, and IL2 Choi et al. [31]. This demonstrates the implication of all metabolites in anti-inflammation pathways. In contrast [36], reports that polycose extract from pumpkin can increase the weight of the immune function of mice as well as the phagocytic function of the macrophages.
Additionally, a preliminary antimicrobial screening of C. maxima seed extracts demonstrated inhibitory effects against Staphylococcus aureus (gram+), Pseudomonas aeroginosae ATCC53 (gram-), and E. coli B12 (gram-), in line with earlier reports on pumpkin (Cucurbita pepo L.) seed oil [32].
The total phenolic content ranged from 627.62±0.1 to 790.54±0.019 mg of gallic acid per gram of extract. The order of total phenolic content was EtOAc > EtOH > Water (Table 5), echoing the findings of [6], who examined a variety of pumpkin seeds and identified high content and diversity of polyphenols. In another study by Pazinato et al. [33], the phenolic compounds content was shown to be between 611.39 and 1053.18 (mg GAE per 100 g of oil). Furthermore, the flavonoid content in our study was lower than that reported by [31] for C. maxima juices.
The antioxidative effect results were congruent with the research of Nawirska-Olszańska et al. [6], who examined the antioxidative effect of the seeds of 12 pumpkin varieties belonging to the species Cucurbita maxima Duch. and Cucurbita pepo L. The seeds of C. pepo exhibit better antioxidant properties regardless of the extraction solvent used. The same result was found for other studies [33], for which the IC50 values were 554.2 to 788.8 (μg per ml). For the oil extracted from the mixture (seeds/peel), while our results were superior to those obtained by Rezig et al. [3], 36.22% of DPPH radicals were quenched by pumpkin seed oil. In addition, two varieties of pumpkin seed oils were potentially active. IC50 of indigenous pumpkin seed oil (IPSO) and hybrid pumpkin seed oil (HPSO) were observed as 3.72 ± 0.93 μg/mL and 5.73 ± 0.96 μg/mL, respectively [30].
In summary, our findings align with previous studies, providing further the remarkable antioxidant properties of C. maxima seeds and the potential for photoprotection offered by the EtOAc extract which is in line with our previous study [10]. This photoprotection aspect deserves further investigation. Currently, this article uses in vitro tests, and future research will include in vivo tests to supplement it.
CONCLUSION
In conclusion, our research findings underscore the immune-enhancing potential of aqueous C.maxima extract, manifesting through the stimulation of spleen cell proliferation, complement activity, thymic cell function, and cytotoxicity. These results suggest the potential utility of aqueous Cucurbita extract for boosting both humoral and cellular immunity, along with lymphocyte toxicity modulation across various diseases. Furthermore, these extracts exhibit substantial antioxidant and antimicrobial activities, in line with their high total phenolic and flavonoid content. Additionally, their capacity for UV ray absorption enhances their potential as photoprotective agents.
LIST OF ABBREVIATIONS
| CRBC | = Chicken Red Blood Cells |
| FT-IR | = Fourier-transform Infrared Spectroscopy |
ETHICAL STATEMENT
All experimental procedures adhered to ethical regulations and followed animal care guidelines stipulated by the local Ethics Committee CEFST. under reference no. 13/2021/CEF51 morocco.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data and supportive information are available within the article.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


