RESEARCH ARTICLE
Tuberculosis Preventive Behaviors and their Determining Factors among Household Contacts of Tuberculosis Patients in Thailand: A Cross-sectional Study
Mullika Phadoongmai1, 2, Wutthichai Jariya1, *
Article Information
Identifiers and Pagination:
Year: 2024Volume: 17
E-location ID: e18749445283184
Publisher ID: e18749445283184
DOI: 10.2174/0118749445283184240111074905
Article History:
Received Date: 19/09/2023Revision Received Date: 27/12/2023
Acceptance Date: 04/01/2024
Electronic publication date: 17/01/2024
Collection year: 2024

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background
Tuberculosis (TB) places a substantial burden on global public health, particularly in developing countries—including Thailand. Household contacts (HHCs) of individuals infected with TB are considered high risk due to their elevated risk of catching and transmitting the disease.
Objectives
This study explores TB preventive behaviors and their determining factors among HHCs of TB patients.
Methods
A cross-sectional study of 245 HHCs who cared for TB patients was conducted in 2022. A questionnaire was used to collect data, which were analyzed using descriptive statistics and stepwise multiple regression analysis.
Results
The overall mean score for all the TB preventive behaviors combined was high (3.84 ± 0.692). On examining the three domains of TB preventive behaviors, the mean score for healthy self-care behaviors was lower (3.45 ± 0.752) than the mean score for behaviors performed to contain and prevent the spread of TB infection from TB patients (4.09 ± 0.860), as well as that for environmental management behaviors (3.99 ± 0.910). TB preventive behaviors were determined to a statistically significant degree by the response efficacy of TB prevention (b = 0.396, P-value < 0.001), perceived self-efficacy at TB prevention (b = 0.260, P-value = 0.01), and gender (b = −0.146, P-value = 0.047).
Conclusion
Overall, TB preventive behaviors among HHCs are at a commendable level in Thailand. However, healthcare institutions and relevant agencies need to actively promote healthier self-care behaviors within these HHCs. This advocacy should place a strong emphasis on enhancing conviction in the benefits of TB preventive behaviors and confidence in performing these behaviors, especially among male HHCs.
1. INTRODUCTION
Tuberculosis (TB) continues to pose a significant danger to global public health [1]. Although TB is preventable and curable, over 10 million new cases are reported globally each year. TB kills 1.5 million people annually, making it the leading infectious killer worldwide [2]. It was projected that 10.6 million individuals would become ill with TB, and 1.6 million would die from TB in 2021, compared to 10.1 million and 1.5 million, respectively, in 2020 [1]. Although the number of TB infections in Thailand declined gradually between 2000 and 2021, the disease remains a top public health concern [3]. Thailand was still on the list of the top 30 high-TB burden countries [1] and had a TB prevalence rate of 143 cases per 100,000 people as of 2021 [3].
In the general population, various factors associated with elevated TB infection rates include high bacterial load, recent immunosuppression (e.g., HIV, immune disorders, immunosuppressive treatment), malnutrition, diabetes, chronic renal issues, transplantation, silicosis, and tobacco/alcohol use [4]. Close contact with a confirmed TB patient has also been identified as one of the vital contributing factors to the incidence of TB [5, 6].
An individual living in close contact with a TB patient is considered a household contact (HHC) [5, 7, 8]. HHCs have been demonstrated to be at three times the risk of contracting TB than non-HHCs (AOR = 3.00; 95% CI: 1.60, 5.62) [9]. Because HHCs are at a significant risk of TB infection, they should be systematically screened for TB [10]. A previous study conducted in South India reported a latent TB infection prevalence among HHCs of 52.6% (95% CI: 50.1–55.1%) [11]. In addition, a systematic review and meta-analysis of 95 studies involving participants from low- and middle-income settings revealed the prevalence of active TB and latent TB infection among HHCs to be 3.1% (95% CI 2.1–4.5%, I2 = 98.8%) and 45.4% (95% CI 40.7–50.2%, I2 = 98.4%), respectively [12]. The TB incidence rate was highest in the first year following exposure [12]. The prevention of TB in high-risk populations, especially HHCs, is essential for reducing the overall incidence of TB [13].
The promotion of TB preventive behaviors is one of several critical strategies for preventing TB infection among HHCs [14]. HHCs should embrace specific behaviors with the following goals: (1) promoting self-care practices, (2) mitigating transmission of infection from TB patients, and (3) efficiently managing the household environment [14, 15]. Based on the existing literature, few studies have investigated TB preventive behavior and its determinants among HHCs. However, several studies have explored TB preventive behaviors among various other populations. TB prevention behavior among Japanese adults has been found to be determined by self-efficacy and health beliefs, including perceived susceptibility to illness and the perceived severity of the illness [16]. In Thailand, self-efficacy is a statistically predictive factor of TB prevention behavior among village health volunteers in the community [17]. In Bangladesh, TB attendants' preventive behavior was related to their perceived susceptibility, perceived benefits, perceived barriers, and perceived severity of the illness [18].
The factors found to determine TB preventive behaviors in previous studies [16-18] are consistent with protection motivation theory (PMT) [19, 20], which plays a key role in understanding and predicting health behaviors. PMT demonstrates that an individual's intention to adopt a recommended behavior is shaped by two concurrent cognitive processes: threat appraisal and coping appraisal [19, 20]. Threat appraisal comprises perceived vulnerability (i.e., the likelihood of developing the disease) and perceived severity regarding the seriousness of the health threat. Coping appraisal encompasses response efficacy, which denotes an individual's anticipation to adhere to a recommended behavior, and self-efficacy, which is indicative of an individual's confidence in successfully performing specific behaviors [19, 20].
Based on our current understanding, although PMT has been widely applied to various health-related behaviors, there is limited research exploring its relevance to TB preventive behaviors among HHCs. Therefore, this study employed PMT as a framework with the aims to investigate TB preventive behaviors and their determining factors among HHCs of TB patients in Thailand. The findings of this study offer valuable insights for planning future TB preventive strategies for reducing the risk of TB transmission among HHCs.
2. MATERIALS AND METHODS
2.1. Study Settings
The Ministry of Public Health of Thailand has organized the 76 provinces of the country into 13 health service regional networks known as “Health Region” to enhance the effective management and allocation of healthcare resources [21]. Phichit Province, located in the lower northern part of Thailand, is one of five provinces consolidated into Health Region 3. There has been a consistent annual increase in the number of pulmonary TB cases in Phichit Province, with the figures for 2019, 2020, and 2021 standing at 488, 587, and 544, respectively. The TB treatment success rates for these years were 77.05%, 73.94%, and 75.92%, respectively, falling short of the key performance indicator, as TB treatment success rates should ideally exceed 88%. Similarly, the mortality rates during the same period were significantly higher than the target performance indicator of <5%, reaching 12.50%, 16.87%, and 15.26%, respectively [22]. This information reflects the challenging TB situation in Phichit Province.
2.2. Study Design and Study Population
In this cross-sectional study, data were collected on HHCs of TB patients. The study population comprised a total of 544 HHCs primarily tasked with the care of index cases diagnosed with pulmonary TB by a qualified medical practitioner and subsequently registered in 2021 for treatment at TB clinics in public hospitals within Phichit Province [22]. The G*Power program was used to calculate the sample size. To ensure adequate data quality for a multiple regression analysis, a power value of 0.99, an effect size value of 0.15, and an alpha value of 0.05 were considered [23]. Based on 10 independent variables, the G*Power software recommended a sample size of 226. After including a non-response rate of 10%, 249 was the required sample size for this study.
The HHCs were selected using a systematic random sampling approach. Initially, we acquired a list with the names of all 544 pulmonary TB index cases in Phichit Province from the National Tuberculosis Information Program [22]. To begin the sampling process, we randomly selected the first HHC of an index case using a simple random sampling technique. Subsequently, we determined the sampling interval by dividing the total number of HHCs by the required sample size for this study. This sampling interval was then used to sequentially select the subsequent index cases of HHCs for inclusion in the study. The inclusion criteria for HHCs in this study included having shouldered the primary responsibility for caring for an index case for at least two months, being at least 20 years of age, and expressing willingness to participate. The exclusion criterion was being diagnosed with TB.
2.3. Assessment Instruments
The questionnaire employed in this study has three sections. The first section comprises six closed-ended items designed to elicit sociodemographic information: gender, age, educational attainment, income adequacy, medical conditions, and relationship to a TB patient. The second section was constructed based on the theoretical framework of PMT [19, 20], and includes four subsections addressing four principal domains. The first domain pertains to the perceived severity of TB infection, and its subsection includes 10 items. The second domain examined concerns perceived vulnerability to TB infection, with its subsection featuring 13 items. The third domain has perceived self-efficacy at TB prevention as its focus, addressed by 14 items. The fourth domain is the response efficacy of TB prevention, and its subsection consists of 12 items. The third section comprises 20 items focused on TB preventive behaviors categorized into host, agent, and environment domains [15]. These behaviors encompass practices related to healthy self-care behaviors (host), behaviors performed to contain and prevent the spread of TB infection from TB patients (agent), and environmental management behaviors (environment) [14, 15].
The second section of the questionnaire assesses the levels of participants’ agreement using a five-point Likert scale, ranging from 1 (for strongly disagree) to 5 (for strongly agree). A five-point Likert scale ranging from 1 (for never practiced) to 5 (for practiced regularly) was also employed in the third section to measure the frequency of participants’ behaviors. To categorize mean scores into three equally sized levels [24], mean scores within the range of 1.00 to 2.33 were classified as low, while those in the 2.34 to 3.67 range were categorized as moderate, and those falling between 3.68 and 5.00 were considered high.
The content validity of the questionnaire was evaluated in consultation with three experts from different backgrounds. One expert is an academic specializing in research on the prevention of communicable diseases, another is a physician practicing at a public hospital, and the third is a public health officer at the Department of Emerging and Re-emerging Infectious Diseases of the Provincial Public Health Office. To assess content validity, the experts examined the questionnaire items and calculated the item–objective congruence values, which ranged from 0.67 to 1.00. The item–objective congruence values greater than 0.5 were considered acceptable and indicative of a high level of agreement among the experts [25]. After achieving consensus among the experts, a pilot study was conducted using a sample of 30 HHCs who live in Nakhon Sawan Province, which is in Health Region 3, the same region as Phichit Province. The reliability of the questionnaire was assessed during the pilot study. The Cronbach’s alpha coefficients were as follows: 0.739 for perceived severity of TB infection, 0.919 for perceived vulnerability to TB infection, 0.867 for perceived self-efficacy at TB prevention, 0.784 for response efficacy of TB prevention, and 0.863 for TB preventive behaviors. The coefficient values were all greater than 0.700 [26], indicating that the questionnaire possesses strong internal consistency and reliability for this study.
2.4. Data Collection
This study received ethical approval from the Human Research Ethics Committee of Naresuan University, granted under certificate of approval (COA) No. 062/2021 and institutional review board (IRB) clearance No. P3-0152/2563. Data were collected from February 2022 to July 2022 using the questionnaire. The researchers sought and obtained permission from the director of the Phichit Public Health Provincial Office, and from the administrators of each TB clinic in the public hospitals within Phichit Province. To ensure the accuracy and consistency of the data, the researchers conducted a half-day training session for five research assistants involved in the study: one nurse and four public health officers. This training aimed to acquaint the research assistants with the content of the questionnaire, the proper procedures for data collection, and the rights and privacy of the study participants. The research assistants contacted each prospective participant via telephone, provided a comprehensive explanation of the research objectives, and requested their decision on whether to participate. Those who agreed were subsequently consulted to determine their preferred time and location for data collection. Each participant expressed their consent by signing informed consent forms. Subsequently, the research assistants retrieved the questionnaires from a total of 249 HHCs and forwarded them to the researchers for data analysis. The researchers excluded four questionnaires from the data analysis due to incompleteness. Consequently, the final set of questionnaires utilized in the data analysis numbered 245.
2.5. Data Analysis
The independent variables included six sociodemographic variables and four variables related to perceptions surrounding TB infection and prevention. The dependent variable was the TB preventive behaviors of HHCs. Descriptive statistics, including frequency, percentage, mean, and standard deviation (SD), were used for both independent and dependent variables. Multivariate linear regression using the stepwise method was employed to examine the impact of the independent variables on the dependent variable. The underlying assumptions of multiple linear regression analysis were tested [27] before conducting the analysis, and all assumptions were met. The normality of the residuals was assessed using a probability–probability (P–P) plot, and it showed no significant deviations from the expected straight-line pattern of normality. The Durbin–Watson value was 1.661, which is within the 1.50–2.50 range, indicating no autocorrelation between the residuals. Tolerance values exceeded 0.2, and variance inflation factor (VIF) values were below 10.0, indicating that there was no multicollinearity between the independent variables. Scatter plots revealed a symmetrical distribution, indicating that there was no homoscedasticity in the residuals. Data analysis was conducted using the IBM Statistical Package for the Social Sciences (SPSS) software, version 22. Statistical significance was established at a significance level of <0.05.
3. RESULTS
Seventy (28.6%) males participated in this study, while the other participants were female (71.4%). The average age is 49.27 years, with a standard deviation of 15.425. The ages range from a minimum of 20 to a maximum of 93. The largest age group was 60 years and above, comprising 62 individuals (25.3%). Most participants had received primary education, with 120 individuals (49.0%) falling into this category. Regarding income adequacy, 129 participants (52.7%) reported having enough income to cover their living expenses. Seventy participants (29.8%) reported having a medical condition. With respect to their relationship to a TB patient or patients, a significant percentage of the participants, 71.0% (174), were identified as family members with blood or marital ties (e.g., parents, spouse, children). These findings are presented in Fig. (1).
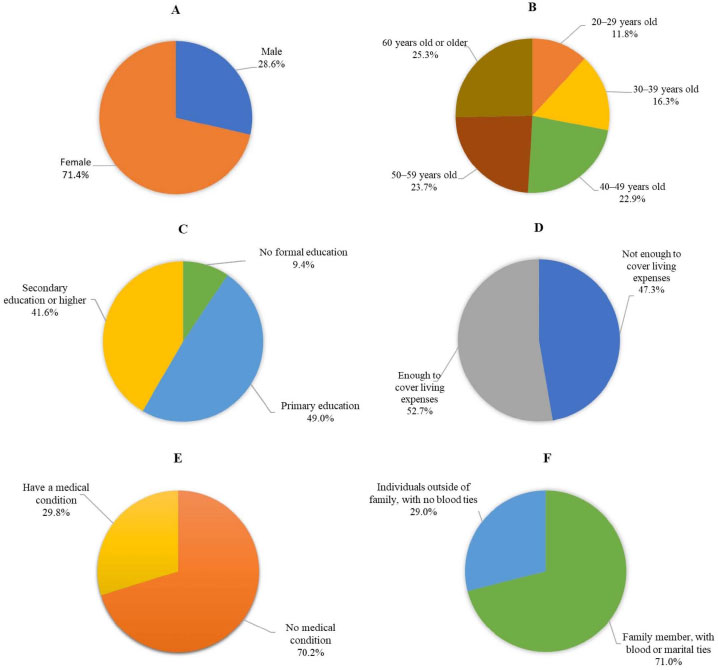 |
Fig. (1). HHCs’ sociodemographic information; (A) = Gender, (B) = Age, (C) = Educational attainments, (D) = Income adequacy, (E) = Medical conditions, (F) = Relationship to a TB patient. |
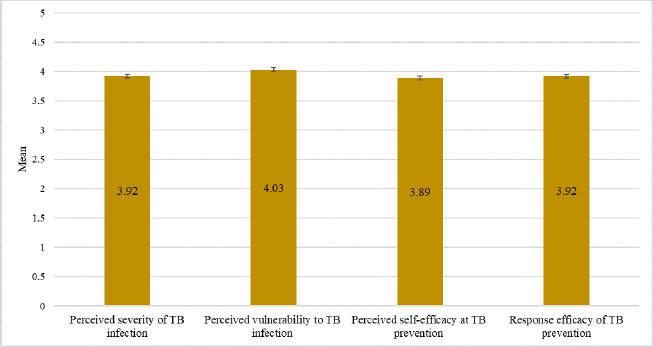 |
Fig. (2). HHCs’ perceptions surrounding TB infection and prevention. |
Fig. (2) presents data on the perceptions surrounding TB infection and prevention held by the study participants. The highest mean score (4.03 ± 0.683) recorded was for perceived vulnerability to TB infection. The mean score for the perceived severity of TB infection (3.92 ± 0.705) was identical to that for the response efficacy of TB prevention (3.92 ± 0.751). Notably, the mean score for perceived self-efficacy at TB prevention (3.89 ± 0.709) was the lowest.
Fig. (3) displays data on the TB preventive behaviors of HHCs in this study. The overall mean score for all the TB preventive behaviors combined was high (3.84 ± 0.692). On examining the three domains of TB preventive behaviors, the mean score for healthy self-care behaviors was lower (3.45 ± 0.752) than the mean score for behaviors performed to contain and prevent the spread of TB infection from TB patients (4.09 ± 0.860), as well as that for environmental management behaviors (3.99 ± 0.910). In addition, of the healthy self-care behaviors assessed, the lowest mean score (2.93 ± 1.359) was for the questionnaire item about undergoing TB screening every six months (Fig. 4).
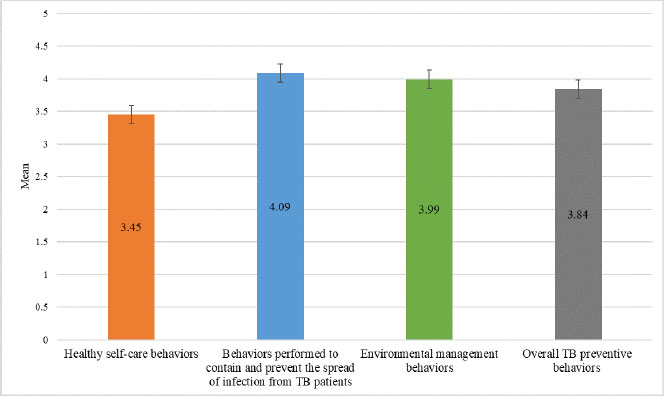 |
Fig. (3). HHCs’ TB preventive behaviors by domains and overall. |
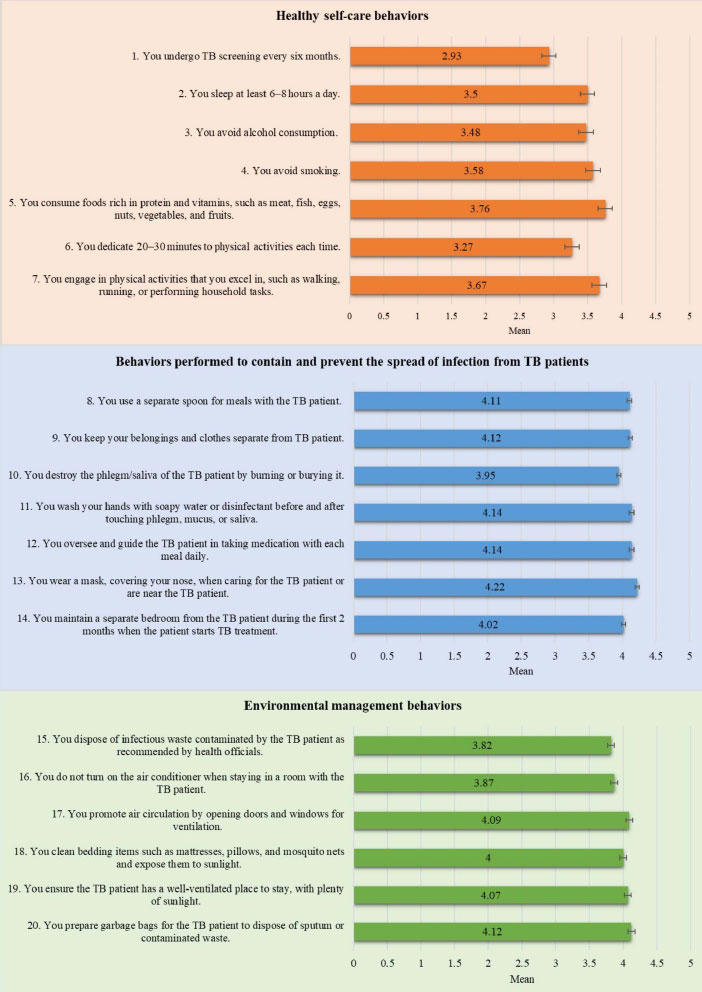 |
Fig. (4). HHCs’ TB preventive behaviors by items. |
| Independent Variable | R Square Change | b | 95%CI for b | β | t | P-value |
|---|---|---|---|---|---|---|
| Response efficacy of TB prevention | 0.416 | 0.396 | 0.251–0.540 | 0.429 | 5.384 | <0.001* |
| Perceived self-efficacy at TB prevention | 0.029 | 0.260 | 0.106–0.414 | 0.267 | 3.331 | 0.001* |
| Male (Reference group = Female) | 0.009 | −0.146 | −0.290–−0.002 | −0.095 | −1.992 | 0.047* |
| Constant (a) = 1.319, R square = 0.454, Adjusted R square = 0.447, F = 66.735, P-value < 0.001 | ||||||
*significant value < 0.05, tested using multiple linear regression analysis.
The results of the multiple linear regression analysis in Table 1 reveal that TB preventive behaviors are determined to a statistically significant degree by the response efficacy of TB prevention (b = 0.396, P-value < 0.001), perceived self-efficacy at TB prevention (b = 0.260, P-value = 0.01), and gender (b = −0.146, P-value = 0.047). These three variables collectively explain 45.4% (R square = 0.454) of the variance in predicting TB preventive behaviors Fig. (5).
4. DISCUSSION
4.1. TB Preventive Behaviors among HHCs
The results of this study shed light on various TB preventive behaviors among HHCs. These behaviors are categorized into three domains: healthy self-care behaviors, behaviors performed to contain and prevent the spread of TB infection from TB patients, and environmental management behaviors [14, 15]. The collective average score for all TB preventive measures was notably high, with a mean of 3.84 ± 0.692. These findings reflect the effectiveness of TB policies and awareness initiatives aimed at TB prevention among HHCs in Thailand [28] and align with those of a prior study conducted in Bangladesh, in which approximately 43.6% of the participants demonstrated a high level of TB preventive behaviors [18].
Notably, the mean score (X̅ = 3.45 ± 0.752) for the healthy self-care behaviors domain was moderate. In particular, the practice of undergoing TB screening every six months was the least commonly performed behavior (X̅ = 2.93 ± 1.359). One plausible explanation for this observation in our study is that a substantial proportion of the participants (49.0%) were aged 50 or older, and nearly half (47.3%) reported having insufficient income to cover their living expenses. Therefore, accessing TB screening services at healthcare facilities might be challenging due to their age and financial constraints. In view of this, a prior study conducted in Thailand proposes that providing financial support to HHCs for transportation should lead to higher TB detection rates [29]. A study conducted in Vietnam found that the contact screening of HHCs is primarily dependent on self-referral or passive methods. Their recommendation is to explore active contact screening with scheduled follow-ups [30]. Another study investigated TB screening coverage among HHCs in Southern Thailand, and their findings reveal a screening coverage rate of 46.6% among their 174 HHC participants. Interestingly, about 20% of these HHCs did not receive any TB screening recommendations [31]. Additionally, HHCs were more likely to undergo TB screening when advised by a healthcare professional than by someone outside that profession [31]. Therefore, the challenges surrounding TB screening among HHCs may require highly targeted interventions and the involvement of healthcare professionals.
4.2. Factors Determining TB Preventive Behaviors among HHCs
In this study, we found that the mean scores for the variables on HHCs’ perceptions regarding TB infection and prevention were high across all four domains: perceived severity of TB infection, perceived vulnerability to TB infection, perceived self-efficacy at TB prevention, and response efficacy of TB prevention. This finding aligns with the findings of another study, which found that a significant majority of TB patient attendants reported a high level of perceived susceptibility (76.1%), a high level of perceived severity of the disease (42.6%), a high level of perceived benefit from TB prevention behaviors (55.3%), and a high level of perceived barriers to performing these behaviors (40.1%) [18]. Furthermore, we found that TB preventive behaviors are determined to a statistically significant degree by the response efficacy of TB prevention (b = 0.396, P-value < 0.001) and perceived self-efficacy at TB prevention (b = 0.260, P-value = 0.01). This indicates that when HHCs have an in-depth understanding of the advantages of TB prevention behaviors and confidence in their ability to prevent TB infection, the frequency of their TB preventive behaviors tends to increase. This finding is consistent with that of a previous study conducted in Bangladesh, which found that response efficacy or perceived benefit is positively correlated with preventive behavior (r = 0.179, P-value < 0.05) [18]. Furthermore, this finding is consistent with the findings of a study conducted in Thailand, which indicates that self-efficacy in TB prevention is statistically predictive of TB-prevention behavior among village health volunteers in the community (β = .244, P-value < .05) [17]. A previous meta-analysis research on PMT argues that perceived self-efficacy and response efficacy are vital components of the coping appraisal process, which assesses an individual’s ability to handle and prevent potential threats [32], such as TB infection of HHCs in our study. Response efficacy refers to the belief that an adaptive response will yield positive results, indicating that taking protective actions will effectively safeguard oneself or others. On the other hand, perceived self-efficacy refers to an individual’s perception of their capability to execute an adaptive response [19, 20, 32]. Self-efficacy is recognized as a crucial element of any health behavior theory, as it plays a pivotal role in regulating motivational, cognitive, and emotional processes [32]. Hence, based on the results of our study, we recommend that healthcare institutions and relevant agencies responsible for equipping and training HHCs should facilitate HHCs recognizing their abilities and competence at TB prevention.
This study found that male HHCs were more likely than female HHCs to engage in risky TB preventive behaviors that could increase the likelihood of TB infection. This finding is consistent with those of previous studies conducted in diverse TB burden settings [5, 33-35]. TB infection rates are much higher among men than among women, as indicated by a male-to-female ratio of 1.7 for worldwide case reports [33]. A study on the risk factors for TB among HHCs in Vietnam reports that males are nearly 1.5 times more likely than females to develop TB [5]. It is unclear and widely discussed in the literature why gender-specific susceptibility exists and the reason is probably highly complex. Sociocultural roles and behaviors likely contribute to higher TB rates in men than in women [33, 36]. For instance, studies conducted in high-burden countries reveal correlations between smoking and the chances of contracting TB—and men are more likely to smoke than women [37, 38]. Similarly, alcohol consumption is linked to an elevated risk of TB, with men being more likely to drink than women [4]. Through a systematic review and meta-analysis, a study has highlighted that men in low- and middle-income countries are disadvantaged and face challenges in their endeavors to seek and obtain TB care in diverse settings [35]. Another study, which investigated risk factors for testing and evaluating close contacts of TB, found that males were significantly more likely than females to not be appropriately evaluated [39]. Therefore, the role of gender in susceptibility to TB may be attributable to gender-based social interactions and behaviors that may enhance exposure to TB and thus raise the risk of contracting the disease [40].
5. LIMITATIONS
Although cross-sectional studies can identify associations between variables, they cannot confirm causal relationships or demonstrate how variables change over time. Therefore, experimental, or longitudinal designs are typically necessary. In addition, because this study covers only one province, the findings may not be generalizable to the entire country or its other provinces. Furthermore, the conclusions drawn from this research may not apply to other geographic regions with potentially different demographics, cultures, or healthcare systems.
CONCLUSION
This study highlights both the strengths and areas for improving TB preventive behaviors among HHCs. Overall, TB preventive behaviors among HHCs are at a commendable level. However, healthcare institutions and relevant agencies should actively promote healthier self-care practices among HHCs, with a specific focus on regular TB screenings. This advocacy should place a strong emphasis on enhancing conviction in the benefits of TB preventive behaviors and confidence in performing these behaviors, especially among male HHCs.
AUTHORS’ CONTRIBUTIONS
W.J. conceptualized the research aim and design. W.J. and M.P. developed the research methodology. M.P. gathered the data. W.J. and M.P. participated in the data analysis and interpretation. M.P. drafted the initial manuscript and W.J. revised, visualized, and wrote up the manuscript. The final manuscript was read and approved by all authors.
LIST OF ABBREVIATIONS
| HHCs | = Household Contacts |
| PMT | = Protection Motivation Theory |
| TB | = Tuberculosis |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The Institutional Review Board of Naresuan University in Thailand granted approval for this study (COA No. 062/2021 and IRB No. P3-0152/2563).
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Informed consent was obtained from all participants.
STANDARDS OF REPORTING
STROBE guidelines were followed.
AVAILABILITY OF DATA AND MATERIALS
Data supporting the results of this study are available upon reasonable request from the corresponding author [W.J.].
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors would like to express sincere gratitude to the HHCs for their participation and cooperation in this study.
REFERENCES
| [1] | Bagcchi S. WHO’s global tuberculosis report 2022. Lancet Microbe 2023; 4(1): e20. |
| [2] | Lönnroth K, Castro KG, Chakaya JM, et al. Tuberculosis control and elimination 2010–50: Cure, care, and social development. Lancet 2010; 375(9728): 1814-29. |
| [3] | The Workd Bank. Incidence of tuberculosis (per 100,000 people) - Thailand 2021. 2021. Available from: https://data.worldbank.org/indicator/SH.TBS.INCD?locations=TH (Accessed on: May 25, 2022). |
| [4] | Narasimhan P, Wood J, MacIntyre CR, Mathai D. Risk factors for tuberculosis. Pulm Med 2013; 2013: 1-11. |
| [5] | Velen K, Nhung NV, Anh NT, et al. Risk factors for Tuberculosis (TB) among household contacts of patients with smear-positive TB in 8 provinces of Vietnam: A nested case-control study. Clin Infect Dis 2021; 73(9): e3358-64. |
| [6] | Tornee S, Kaewkungwal J, Fungladda W, Silachamroon U, Akarasewi P, Sunakorn P. Risk factors for tuberculosis infection among household contacts in Bangkok, Thailand. Southeast Asian J Trop Med Public Health 2004; 35(2): 375-83. |
| [7] | Ai JW, Ruan QL, Liu QH, Zhang WH. Updates on the risk factors for latent tuberculosis reactivation and their managements. Emerg Microbes Infect 2016; 5(1): 1-8. |
| [8] | Ross JM, Xie Y, Wang Y, et al. Estimating the population at high risk for tuberculosis through household exposure in high-incidence countries: A model-based analysis. EClinicalMedicine 2021; 42: 101206. |
| [9] | Shimeles E, Enquselassie F, Aseffa A, et al. Risk factors for tuberculosis: A case–control study in Addis Ababa, Ethiopia. PLoS One 2019; 14(4): e0214235. |
| [10] | WHO consolidated guidelines on tuberculosis Module 2: screening-systematic screening for tuberculosis disease 2021. |
| [11] | Krishnamoorthy Y, Ezhumalai K, Murali S, et al. Prevalence and risk factors associated with latent tuberculosis infection among household contacts of smear positive pulmonary tuberculosis patients in South India. Trop Med Int Health 2021; 26(12): 1645-51. |
| [12] | Fox GJ, Barry SE, Britton WJ, Marks GB. Contact investigation for tuberculosis: A systematic review and meta-analysis. Eur Respir J 2013; 41(1): 140-56. |
| [13] | A situational analysis of programmatic management of TB preventive treatment in the WHO South-East Asia Region 2020. |
| [14] | WHO policy on TB infection control in health-care facilities, congregate settings and households 2009. |
| [15] | van Seventer JM, Hochberg NS. Principles of infectious diseases: Transmission, diagnosis, prevention, and control 2017; 22-39. |
| [16] | Yoshitake N, Omori M, Sugawara M, Akishinonomiya K, Shimada S. Do health beliefs, personality traits, and interpersonal concerns predict TB prevention behavior among Japanese adults? PLoS One 2019; 14(2): e0211728. |
| [17] | Wicharit L, Nakagasien P, Krainuwat K. Predicting factors of Tuberculosis preventive behaviors in community among village health volunteers, Udon Thani Province. J Popul Soc Stud 2022; 30: 703-25. |
| [18] | Jahan F, Rattanagreethakul S, Toonsiri C. Factors related to Tuberculosis preventive behaviors among Tuberculosis patient attendants in Dhaka, Bangladesh. Thai Pharm Heal Sci J 2014; 9: 68-74. |
| [19] | Prentice-Dunn S, Rogers RW. Protection motivation theory and preventive health: Beyond the health belief model. Health Educ Res 1986; 1(3): 153-61. |
| [20] | Rogers RW. Cognitive and physiological processes in fear appeals and attitude change: A revised theory of protection motivation 1983. |
| [21] | Nundy M, Bhatt P. The health system in the Kingdom of Thailand: Reforms, achievements, and challenges (CSEP working paper 45) s 2022. |
| [22] | Bureau of Tuberculosis. National Tuberculosis Information Program 2021 2021. Available from: https://ntip-ddc.moph.go.th/uiform/ProgramUpdate.aspx (Accessed on: January 12, 2022). |
| [23] | Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav Res Methods 2009; 41(4): 1149-60. |
| [24] | Watson Todd R. Analyzing and interpreting rating scale data from questionnaires. Reflections 2018; 14: 69-77. |
| [25] | Rovinelli RJ, Hambleton RK. On the use of content specialists in the assessment of criterion-referenced test item validity. Tijdschr Voor Onderwijsres 1977; 2: 49-60. |
| [26] | Taber KS. The use of Cronbach’s Alpha when developing and reporting research instruments in science education. Res Sci Educ 2018; 48(6): 1273-96. |
| [27] | Hair JF, Black WC, Babin BJ, Anderson RE, Tatham RL. Multivariate data analysis 6th ed. 2006. |
| [28] | Department of Disease Control Ministry of Public Health. Thailand operational plan to end tuberculosis 2017–2021. 2018. Available from: https://tbthailand.org/download/Manual/Thailand Operational Plan To End TB_2017_2021.pdf (Accessed on: December 12, 2022). |
| [29] | Imsanguan W, Bupachat S, Wanchaithanawong V, et al. Contact tracing for tuberculosis, Thailand. Bull World Health Organ 2020; 98(3): 212-8. |
| [30] | Thanh THT, Ngoc SD, Viet NN, et al. A household survey on screening practices of household contacts of smear positive tuberculosis patients in Vietnam. BMC Public Health 2014; 14(1): 713. |
| [31] | Oo MM, Tassanakijpanich N, Phyu MH, et al. Coverage of tuberculosis and diabetes mellitus screening among household contacts of tuberculosis patients: A household-based cross-sectional survey from Southern Thailand. BMC Public Health 2020; 20(1): 957. |
| [32] | Floyd DL, Prentice-Dunn S, Rogers RW. A meta-analysis of research on protection motivation theory. J Appl Soc Psychol 2000; 30(2): 407-29. |
| [33] | Hertz D, Schneider B. Sex differences in tuberculosis. Semin Immunopathol 2019; 41(2): 225-37. |
| [34] | Wada PY, Costa AG, Araújo-Pereira M, et al. Possible sex difference in latent tuberculosis infection risk among close tuberculosis contacts. Int J Infect Dis 2022; 122: 685-92. |
| [35] | Horton KC, MacPherson P, Houben RMGJ, White RG, Corbett EL. Sex differences in Tuberculosis burden and notifications in low- and middle-income countries: A systematic review and meta-analysis. PLoS Med 2016; 13(9): e1002119. |
| [36] | Mlondo MN, Melesse SF, Mwambi HG. Risk factors associated with Tuberculosis among men; A study of South Africa. Open Public Health J 2022; 15(1): e187494452202240. |
| [37] | Maurya V, Vijayan VK, Shah A. Smoking and tuberculosis: An association overlooked. Int J Tuberc Lung Dis 2002; 6(11): 942-51. |
| [38] | Bates MN, Khalakdina A, Pai M, Chang L, Lessa F, Smith KR. Risk of tuberculosis from exposure to tobacco smoke: A systematic review and meta-analysis. Arch Intern Med 2007; 167(4): 335-42. |
| [39] | Barroso EG. Factors associated with household contacts’ tuberculosis testing and evaluation. Public Health Nurs 2020; 37(5): 705-14. |
| [40] | Hudelson P. Gender differentials in tuberculosis: The role of socio-economic and cultural factors. Tuber Lung Dis 1996; 77(5): 391-400. |