All published articles of this journal are available on ScienceDirect.
Regular Consumption of Fortified Growing-up Milk Attenuates Upper Respiratory Tract Infection among Young Children in Indonesia: A Retrospective Cohort Study
Abstract
Introduction
A Growing-up Milk (GUM) supplemented with short-chain Galacto-oligosaccharides (scGOS), long-chain Fructo-oligosaccharides (lcFOS), and omega-3 Long-chain Polyunsaturated Fatty Acids (n-3 LCPUFAs) could support the development of the immune system to prevent the Upper Respiratory Tract Infection (URTI) and associated morbidities. It is of interest to assess whether a daily consumption of scGOS/lcFOS/n-3 LCPUFAs-fortified GUM for a minimum of 6 months reduces URTI incidences within the subsequent 3 months among young children in Indonesia.
Methods
A total of 154 children aged 18 to 36 months were recruited in this retrospective cohort study and categorized into exposed and non-exposed groups (78 and 76 children, respectively). A guided interview was conducted with each subject’s parent. Demographic and clinical information was collected, including incidence of URTI within the past three months, duration of URTI, as well as the frequency of medical visits when contracting URTI. The bivariate analysis via chi-square test as well as the multivariate analysis via binary logistic regression were performed.
Results
The overall characteristics were similar between groups with certain distinctions. The bivariate analysis indicated the exposed group to have better protection against URTI within the past 3 months as compared to the non-exposed group (RR=0.62; 95% CI=0.41-0.92; P<0.05). The multivariate analysis reinforced the finding by reporting that the exposed group was indeed protected against URTI (adjusted RR=0.42; 95% CI=0.21-0.85; P<0.05).
Conclusion
A routine consumption of scGOS/lcFOS/n-3 LCPUFAs-fortified GUM for a minimum of 6 months among Indonesian young children protected against URTI up to 58%, suggesting that fortified GUM consumption supports proper development of the immune system.
1. INTRODUCTION
A functional immune system is essential to protect children against multiple pathogens. The immune system, along with other body systems, must develop well from an early age to achieve that purpose [1]. The proper development and functionality of the immune system are profoundly influenced by the relationship between the immune system and gastrointestinal microbiota [2]. The proper development of gastrointestinal microbiota in early life has been shown to be associated with protection against various diseases in adulthood, including asthma, obesity, and intestinal inflammation [2], and that gastrointestinal microbes and their produced metabolites are important in activating various players of the immune system [3-6]. The ecosystem of gastrointestinal microbiota within a host develops from birth and is heavily influenced by maternal (e.g., mother's gastrointestinal microbiota and vaginal infections during pregnancy) and postnatal factors (e.g., diet and antibiotic consumption). The microbiota profile of children gradually resembles the configuration of adults with a peak at 3 years of age [7].
One postulated way to support the proper development of the gastrointestinal microbiota as well as the immune system is by providing a variety of healthy foods to infants and young children. Two healthy foods of interest are prebiotics and n-3 long-chain Polyunsaturated Fatty Acids (LCPUFAs) [8]. Prebiotics are substrates that are selectively utilized by host microorganisms conferring a health benefit [9]. Prebiotics could modulate the host's health status through indirect (via metabolites produced during bacterial fermentation) and direct mechanisms (via interaction between prebiotics and surface receptors expressed by the gut-associated epithelial and innate immune cells) [10]. For example, it has been suggested that prebiotic intake could protect against Upper Respiratory Tract Infection (URTI) because the prebiotic supplementation could provide an anti-inflammatory effect at the site of infection as well as within the circulation [11].
An example of prebiotics is non-digestible oligosaccharides, including galactans and fructans, because they could enrich Lactobacillus and/or Bifidobacterium species, resulting in health benefits [9]. The health benefits of prebiotic intake in infancy have been investigated, either via individual supplementation, such as inulin [12, 13], Galacto-oligosaccharides (GOS) [14], or Fructo-oligosaccharides (FOS) [15-17], or a combination, such as a mixture of short-chain GOS (scGOS) and long-chain FOS (lcFOS) [18-20]. Of note, a specific mixture of scGOS and lcFOS in a ratio of 9:1 has been demonstrated to be similar to the proportion found within the human milk oligosaccharides [21, 22]. Furthermore, this mixture at a dose of 0.4 – 0.8 g/100 mL could modulate the intestinal microbiota and subsequently support immune development [21, 23-25].
Similarly, LCPUFAs are important fatty acids for immune development, and a balanced profile of LCPUFAs could result in optimal immune regulation and activation [26-30]. It is known that an imbalanced ratio of n-6:n-3 LCPUFAs could induce an inflammatory condition [31]. Thus, an increased dietary intake of n-3 LCPUFAs could balance the LCPUFAs ratios and support proper immune development, particularly during infancy or young childhood. Some of the most important n-3 LCPUFAs in the diet are Alpha-linolenic Acid (ALA), Eicosapentaenoic Acid (EPA), and Docosahexaenoic Acid (DHA). ALA is the precursor of n-3 LCPUFA, which is converted into EPA and DHA. While ALA is found in linseeds, rapeseed oil, and walnuts, EPA and DHA are found in fatty fish [32].
It has been reported that an intake of Growing-up Milk (GUM) supplemented with scGOS/lcFOS/n-3 LCPUFAs among young children could reduce the risk of upper respiratory tract or gastrointestinal infection [33]. However, the risk reduction was of borderline statistical significance, indicating that similar studies need to be performed [33]. We, therefore, aimed to assess whether young children in Indonesia who consumed scGOS/lcFOS /n-3 LCPUFAs-fortified GUM routinely for a minimum of 6 months would have a better immune system, as indicated by the reduced incidence of URTI in the subsequent 3 months.
2. MATERIALS AND METHODS
The study was a retrospective cohort clinical study conducted across Indonesia, covering all provinces in Java Island and more than ten provinces on other islands, between September and December 2022.
2.1. Ethical Approval
Ethical approval was obtained from the institutional review board and written informed consent was obtained from all participating parents. This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving research study participants were approved by the research ethics committee of the Faculty of Medicine Universitas Katolik Indonesia Atma Jaya, with approval no. 05/11/KEP-FKIKUAJ/2022.
2.2. Study Participants
Children aged 18 to 36 months were recruited by a third-party agency into the study after informed written consents were obtained from the parents. The participants were subsequently categorized into two groups, i.e., the exposed group and the non-exposed group.
The criterion of exposure was daily consumption of a minimum of 500 mL per day of GUM fortified with 0.136 mg/mL of n-3 LCPUFAs (comprising DHA and EPA) and 0.6 g/100 mL of scGOS: lcFOS in a ratio of 9:1 for minimum 6 months. The criteria of the non-exposed group were never consumed GUM, or consumed GUM fortified with scGOS:lcFOS (9:1) and n-3 LCPUFAs for less than 6 months or less than 500 mL per day, as well as consumption of non-scGOS:lcFOS-fortified GUM.
The exclusion criteria were COVID-19 diagnosis by a physician within the past 6 months, tuberculosis, type 1 diabetes, human immunodeficiency virus infection, and any congenital disorder. Subjects with antibiotic and/or corticosteroid use were excluded. The sample size was defined based on the proportion of Nocerino et al.’s study [34] and calculated with OpenEpi version 3.01 [35], reporting that a minimum of 75 children per group were needed to detect a 20% change in the URTI between exposed and unexposed groups with a power of 80% at an alpha level of 5%.
2.3. Data Collection
A guided interview was conducted with each subject’s parent through telephone to practice social distancing during the COVID-19 pandemic. Demographic charac-teristics, including maternal educational status, maternal working status, family size, and number of school-age siblings, were collected. Respective characteristics of the subjects were collected as well, including age, sex, birth history, as well as nutritional and immunization statuses. The immunization status of all children was assessed by evaluating their adherence to the national immunization criteria according to their age, in order to ensure that the immunization status was comparable across all groups. During the phone call, each parent was asked to recall whether the subject developed any symptom of URTI (defined as having either cough and fever, rhinorrhea and fever, or cough and rhinorrhea and fever) within the past three months, duration of URTI, as well as the frequency of medical visit when contracting URTI. Subjects having COVID-19 in the past six months as confirmed clinically by doctors or tests and suffering from known diseases, including tuberculosis, type one diabetes, HIV, and congenital immune disorder, were excluded from the study.
2.4. Outcome Measurement: Upper Respiratory Tract Infection
The outcomes of the study included the frequency and length of URTIs as well as the number of medical visits that occurred during an episode, which were compared between the exposed group (those who consumed scGOS/lcFOS/n-3 LCPUFA-fortified GUM on a daily basis for at least six months) and the non-exposed group while controlling for potentially confounding factors, like the subjects' morbidity, immunization status, and mothers' demographics.
2.5. Statistical Analysis
Data analysis was performed using SPSS (IBM SPSS Statistics for Windows, Version 29.0. Armonk, NY: IBM Corp). Descriptive statistics were used to describe categorical data (i.e., absolute number and percentage) and numerical data (i.e., mean ± standard deviation). The chi-square test was performed to test a statistically significant relationship between variables organized in a bivariate table. The binary logistic regression was performed to determine factors related to URTI, in which the relative risk with a 95% confidence interval was calculated. A difference with P<0.05 was interpreted as statistically significant. Variables with a p-value less than 0.2 in bivariate analysis, or those theoretically known to have a significant impact on the incidence of URTI according to references, were included in the multivariate logistic regression analysis.
3. RESULTS
There were 154 children recruited into this study, comprising 78 and 76 subjects in the exposed and unexposed groups, respectively. The recruitment flow is explained in Fig. (1). Table 1 displays the demographic characteristics of those subjects and their respective mothers. The overall characteristics of the subjects were similar between the two groups, except that in the exposed group, there were more children born by the caesarian section (45; 57.7%) and fewer children of low birth weight (6; 7.7%). These characteristics were not statistically different (P>0.05). In addition, information on the number of school-age siblings was collected, in which there were more children with school-age siblings in the exposed group (22 out of 30 respondents with sibling: 73.3%) than in the non-exposed group (18 out of 33 respondents with sibling: 54.5%), but the difference was not statistically different (P>0.05). There were more mothers with university degrees (58: 74.4%) and more working mothers in the exposed group (59: 75.6%) than in the non-exposed group (38: 50.0% and 44: 57.9%, respectively). These characteristics were statistically different (P=0.004 and P=0.019, respectively). Next, the clinical characteristics of the subjects are shown in Table 1. Information on nutritional and immunization statuses was obtained as they might determine the proper development of the immune system among young children. However, there was no statistical difference observed within the nutritional and immunization statuses between the exposed and unexposed groups (P>0.05).
The bivariate analysis was subsequently performed to assess the impact of routine consumption of scGOS/lcFOS/n-3 LCPUFAs-fortified GUM on contracting URTI within the past 3 months. Information on three aspects was collected, i.e., the incidence and duration of URTI as well as the number of medical visits during an episode of URTI (Table 2). It was observed that subjects within the exposed group had statistically better protection against URTI as compared to the ones in the non-exposed group (RR=0.62; 95% CI=0.41-0.92; P=0.015) with an absolute risk reduction of 19.2% (95% CI= 4.0-34.4%). The exposed group exhibited a shorter duration of URI and fewer visits to the physician during the illness as well, but the differences were not statistically significant (P>0.05).
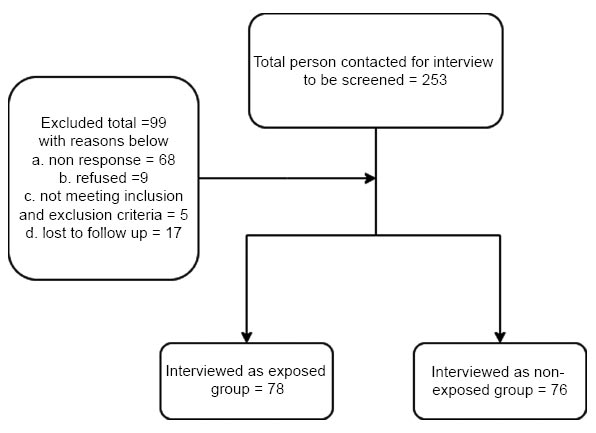
Recruitment flow diagram.
| Characteristics | Exposed (n=78) |
Non-exposed (n=76) | P-value | |
|---|---|---|---|---|
| Mothers | ||||
| Educational status, n (%) | High school completion | 20 (25.6) | 38 (50.0) | 0.004 |
| Graduate | 56 (71.8) | 38 (50.0) | ||
| Postgraduate | 2 (2.6) | 0 (0.0) | ||
| Working status, n (%) | Stay at home | 19 (24.4) | 32 (42.1) | 0.019 |
| Working | 59 (75.6) | 44 (57.9) | ||
| Number of children, median (range) | 1 (1-3) | 1 (1-4) | 0.419 | |
| Children | ||||
| Age (months), mean ± standard deviation | 28.68 ± 4.27 | 29.49 ± 4.17 | 0.237 | |
| Sex, n (%) | Male | 44 (56.4) | 43 (56.6) | 0.983 |
| Female | 34 (43.6) | 33 (43.4) | ||
| History of labour, n (%) | Normal | 33 (42.3) | 37 (48.7) | 0.225 |
| Assisted delivery | 0 (0.0) | 2 (2.6) | ||
| Caesarian section | 45 (57.7) | 37 (48.7) | ||
| Nutritional status, n (%) | Normal | 50 (64.1) | 32 (42.1) | ref. |
| Underweight | 8 (10.3) | 15 (20.5) | 0.079 | |
| Wasting | 20 (25.5) | 29 (39.7) | 0.065 | |
| Immunization status, n (%) | Complete | 62 (79.5) | 58 (76.3) | ref. |
| Incomplete | 16 (20.5) | 18 (23.7) | 0.635 | |
| Low birth weight, n (%) | 6 (7.7) | 11 (14.5) | 0.206 | |
| School-age sibling*, n/respondents (%) | 22/30 (73.3) | 18/33 (54.5) | 0.190 | |
Table 2.
| Group | Incidence of URTI | Duration of URTI | Total Medical Visit during URTI | |||||
|---|---|---|---|---|---|---|---|---|
|
Yes n (%) |
No n (%) |
crude RR (95% CI) |
P-value |
Median (range) |
P-value |
Median (range) |
P-value | |
| Exposed (n=78) | 24 (30.8) | 54 (69.2) | 0.62 (0.41-0.92) |
0.015 | 4 (0-20) |
0.160 | 0 (0-5) |
0.207 |
| Non-exposed (n=76) |
38 (50) | 38 (50) | 5 (0-34) |
1 (0-5) |
||||
| Variable | Upper Respiratory Tract Infection | |||
|---|---|---|---|---|
| Yes, n (%) | No, n (%) | Adjusted RR (95% CI) |
||
| Group | Exposed | 24 (30.8) | 54 (69.2) | 0.42 (0.21 – 0.85) * |
| Non-exposed | 38 (50.0) | 38 (50.0) | ref. | |
| Underweight | Yes | 10 (43.5) | 13 (56.5) | 0.84 (0.31 – 2.29) |
| No | 52 (40.6) | 76 (59.4) | ref. | |
| Wasting | Yes | 23 (46.9) | 26 (53.1) | 1.32 (0.61 – 2.87) |
| No | 39 (38.2) | 63 (61.8) | ref. | |
| Immunization status | Incomplete | 14 (41.2) | 20 (58.8) | 1.19 (0.53 – 2.69) |
| Complete | 48 (40.0) | 72 (60.0) | ref. | |
| Maternal educational status | High school completion | 26 (44.8) | 32 (55.2) | 0.52 (0.03 – 9.40) |
| Graduate | 35 (37.2) | 59 (62.8) | 0.44 (0.03 – 7.63) | |
| Postgraduate | 1 (50.0) | 1 (50.0) | ref. | |
| Maternal working status |
Stay at home | 22 (43.1) | 29 (56.9) | 1.06 (0.51 – 2.21) |
| Working | 40 (38.8) | 63 (61.2) | ref. | |
The multivariate analysis was finally performed to assess variables that might modulate the risk of the exposed group towards contracting URTI. Two statistically significant characteristics (Table 1, i.e., the educational and working statuses of the mothers, were included in the analysis. The nutritional and immunization statuses of the subjects were included as well, despite the differences being not statistically different, because these statuses might influence the adequate maturity of the immune system among young children. Table 3 demonstrates that upon adjustment of the maternal, educational, and working statuses as well as the subjects’ nutritional and immunization statuses, the exposed group retained protection against URTI as compared to the non-exposed group (RR=0.42; 95% CI=0.21-0.85; P=0.017). This suggests that daily consumption of scGOS/lcFOS/n-3 LCPUFAs-fortified GUM for a minimum of 6 months could provide adequate protection against URTI up to 58% within the past 3 months.
4. DISCUSSION
We have hereby reported a retrospective cohort study assessing URTI (within the last 3 months from the time of interview) among Indonesian children aged 18-36 months who consumed a minimum of 500 mL per day of GUM supplemented with 0.6 g/100 mL of scGOS/lcFOS (9:1) and 0.136 mg/mL of n-3 LCPUFAs for at least 6 months. Our retrospective study design has provided a quick and inexpensive way to obtain data [36], in which we chose 6 months of GUM consumption for young children as the minimum period to start benefiting from its health benefits. In addition, the subsequent 3-month recall period was chosen to allow observation as accurately as possible.
We observed that young children within the exposed group, who routinely consumed scGOS/lcFOS/n-3 LCPUFAs-fortified GUM, had a lower relative risk of contracting URTI (RR=0.62; 95% CI=0.41-0.92; P<0.05) as compared to the ones within the non-exposed group. This protective effect was supported by the multivariate analysis upon adjustment of several variables (RR=0.42; 95% CI=0.21-0.85; P<0.05). Those variables have been shown to be associated with the incidence of URTI, including sub-optimum nutritional status [37], incomplete immunization status [38], working mothers [39], as well as low levels of maternal education [40]. Hence, it was interesting to observe that after adjusting those variables, the protective effect of consuming fortified GUM remained. Our findings were partly supported by a previous finding [41], which demonstrated that the intake of infant formula supplemented with 0.4 g/100 mL of scGOS/lcFOS with a ratio of 9:1, as compared to the intake of standard infant formula, resulted in a lower number of URTI episodes and lower antibiotic intake. Furthermore, our findings have reinforced the published result on young children who consumed GUM supplemented with 0.8 g/100 mL of scGOS/lcFOS as well as n-3 LCPUFAs [33], suggesting protection against URTI due to a regular consumption of milk supplemented with certain prebiotics and omega-3 fatty acids.
Prebiotics have been extensively demonstrated to modulate intestinal microbiota and provide health benefits to the host [42, 43]. Breast milk-derived human milk oligosaccharides, the first prebiotics consumed by many infants, could promote the dominance of Bifidobacterium species within the intestinal tract during early life, which supports infant health [44]. It has also been shown that human milk oligosaccharides exhibit antimicrobial activity against gram-positive bacteria, which could prevent skin infection of the mother’s breast and protect infants from respiratory pathogens [45]. Other prebiotics have been reported to be utilized in milk formula as well, including resistant starch, pectin, gums, and oligosaccharides (e.g., xylo-oligosaccharides, gluco-oligosaccharides, GOS, and FOS) [9]. A recent meta-analysis of 17 clinical trials indeed suggested oral prebiotic supplementation in infants and children to significantly reduce the risk of a subject contracting respiratory tract infection [8]. This evidence has enhanced the conclusion of our study. Similarly, De Cosmi et al., Bryan D et al., and Pastor N et al. have declared how prebiotics can enhance the role of inflammatory mediators against infection [46-48]. This could be the underlying biomedical process of our study.
Our study has several limitations. First, there might be a recall bias as the data were collected retrospectively. Second, the data were collected only through guided interviews via phone calls. No clinical examination/visit was conducted to confirm the reported symptoms. Third, the data were collected from children living in the urban setting of particular regional areas in Indonesia. Despite that the subjects have been recruited from the majority of provinces in Indonesia, the sample size calculation has not been representative of the Indonesian population, especially from rural settings.
CONCLUSION
We observed routine consumption of scGOS/lcFOS/n-3 LCPUFAs-fortified GUM among Indonesian children aged 18-36 months old to have a protective effect on URTI compared to those not routinely consuming it. This result suggests that GUM supplemented with certain prebiotics and n-3 LCPUFAs for young children may provide possible protection against respiratory tract infection.
AUTHORS' CONTRIBUTIONS
It is hereby acknowledged that all authors have accepted responsibility for the manuscript's content and consented to itssubmission. They have meticulously reviewed all results and unanimously approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| GUM | = Growing-up Milk |
| scGOS | = Short-chain Galacto-oligosaccharides |
| lcFOS | = Long-chain Fructo-oligosaccharides |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
All procedures involving research were approved by the research ethics committee of the Faculty of Medicine Universitas Katolik Indonesia Atma Jaya, with approval no. 05/11/KEP-FKIKUAJ/2022.
HUMAN AND ANIMAL RIGHTS
No Animals were used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.


