All published articles of this journal are available on ScienceDirect.
SARS-CoV-2 Antibody Level after Homologous and Heterologous Booster Vaccines: an 18-month Longitudinal Observational Cohort Study in Indonesia
Abstract
Background
Recently, there have been reports of the rise of COVID-19 cases in several sites. The effectiveness of the COVID-19 vaccine was reported elsewhere. There are still questions on how the kinetics of antibody response during relatively long periods, the need for additional doses, and the effect of homologous and heterologous boosters. The study was conducted to analyze the kinetics of antibody response after the primary dose and the third dose of the ChAdOx1 vaccine in individuals previously receiving two doses of the ChAdOx1 (homologous) and CoronaVac (heterologous) COVID-19 vaccines.
Methods
The study population comprised 52 men and 98 women, divided into CoronaVac Recipients and ChAdOx1 Recipients for the first two doses according to the recommended schedule by the Ministry of Health of Indonesia (MoH).Six months after the second dose, the third dose of ChAdOx1 was administered as a homologous and heterologous booster. COVID-19 antibody levels were measured by the CMIA method before the first dose (time-point or TP1), two weeks after the first dose (TP2), before the second dose (TP3), 1 month after the second dose (TP4), 12 months after the second dose (TP5), and 18 months (TP6) after the second dose administration. Six months after the second dose, the third dose of ChAdOx1 was administered as a homologous and heterologous booster. Along with these, several epidemiological data were collected from subjects on TP1.
Results
A total of 153 serum samples were collected from subjects who had received the third dose, and the antibody response was measured. On TP1, COVID-19 antibody reactivity (the level was >50 AU/mL) was detected on 100 (66,67%) of subjects, indicating a possible previous exposure to SARS-CoV-2. On TP2, the sharp increase in antibody level was documented in the ChAdOx1 group. However, in the following data during the cohort, the gap was narrowing, and on the TP6, the antibody levels showed no significant difference between groups (p>0.05). Likewise, no significant differences were shown between groups with or without a history of COVID-19 antibody reactivity on TP1 (p>0.05). Considering epidemiological characteristics, no significant differences were documented based on sex, age groups, and BMI level.
Conclusion
This study provides a deeper understanding of the kinetics of antibody levels longitudinally among those with and without previous history of SARS CoV-2 infection, among the recipients of different vaccines, and the recipients of homologous and heterologous boosters. It is necessary to elucidate further in the next study how the level of antibody reflects the neutralizing antibody level as an indicator of protection against the infection risk.
1. INTRODUCTION
The COVID-19 pandemic swept the globe and changed the paradigms of public health and healthcare systems [1, 2]. The global battle against COVID-19 has witnessed fluctuating trends, with periods of decline often followed by resurgences in cases. Recently, the rise of COVID-19 cases was again reported in December 2023 [3] even during May 2024 in Singapore [4] and June 2024 in the United States [5].
Positive cases and deaths from new COVID-19 strains continue to occur even after three years, as evidenced by wastewater monitoring and clinical diagnosis. In addition, some common detrimental mutations are found in SARS-CoV-2 genomes from deceased COVID-19 patients from different continents. It highlights that the risk of COVID-19 is still present; thus, care and preparedness should never be abandoned [6-8].
During pandemic times, COVID-19 vaccination was initiated in December 2020 [9]. ChAdOx1, a non-replicating adenovirus vector encoding the SARS-CoV-2 viral spike protein, is one of the most widely used vaccines. ChAdOx1 appears to be a viable preventive measure against COVID-19 disease based on early clinical trials; however, concerns remain regarding the long-term immune response and the need for repeated doses to maintain protection [10, 11].
Meanwhile, several nations have integrated the CoronaVac vaccine—which uses the inactivated SARS-CoV-2 virus as an antigen—into their immunization regimens. According to a preliminary investigation, there may be a correlation between age and specific health factors and the effectiveness of CoronaVac in avoiding severe COVID-19 disease. As with previous vaccinations, further study is required to comprehend the immune response that grows over time after receiving the first and second doses of CoronaVac [12, 13].
In this situation, the administration of additional doses (booster doses) has been suggested as a strategy to strengthen and sustain the immune response to SARS-CoV-2. There are now two different methods of administering booster shots: homologous boosting, which uses the same vaccine platform for both the primary series and the booster, and heterologous boosting, which uses a separate vaccine platform [14].
Utilizing the same vaccine platform as the primary series, homologous booster vaccines have been extensively researched and put into practice. These vaccinations have proven to be effective in boosting immune responses and offering heightened defense against COVID-19, including its evolving variations. Conversely, heterologous boosting has drawn interest as a possible tactic to expand the immune response and maybe provide improved protection [15].
According to recent research, heterologous boosting might be more successful at eliciting a stronger immune response overall, which could provide greater defense against SARS-CoV-2 subtypes [16]. This strategy might benefit from the distinct immunological pathways and modes of action triggered by multiple vaccination platforms, resulting in a more varied and all-encompassing immune response [17]. The long-term effectiveness of this strategy is currently being assessed despite the encouraging potential benefits of heterologous boosting [18, 19].
Meanwhile, in the general population of previous recipients of two doses of ChAdOx1 nCoV-19, the third dose of ChAdOx1 nCoV-19 (Vaxzevria, Cambridge, Astra Zeneca, UK) demonstrated significant immunogenicity for both the humoral and cellular arms of the immune response [20, 21].
Nevertheless, it is still unknown how the extra dose would impact the immune response in those who have already received the vaccine's primary two doses. Moreover, the effect of ChAdOx1 as a heterologous booster to other vaccines, mainly CoronaVac, is still unclear.
The study evaluated the kinetics of antibody levels during the primary doses and for 18 months after the second dose administration with CoronaVac and ChAdOx1 based on the recommended schedule [22]. It also observes the difference between one with or without a possible previous infection as well as between one receiving a homologous and heterologous third dose as a booster. The understanding will be part of the strategy for monitoring post-pandemic COVID-19 cases. Moreover, it will contribute to developing more tailored and effective vaccination regimens in response to the possible challenges posed by SARS-CoV-2 after the pandemic.
2. METHODS
2.1. Study Design and Participants
The study is a longitudinal cohort design conducted from August 2021 until May 2023, along with the Indonesian Ministry of Health (MoH) vaccination program. The types of vaccines and study sites in the two cities were based on the recommendations of the MoH and the local government [22].
All participants met the inclusion criteria, were 18-59 years old, were not pregnant, and had no record of a formal report or self-report of previous symptomatic infection of COVID-19. The criteria referred to technical guidelines for pre-COVID-19 vaccination recipient screening issued by the Indonesian MoH [23].
The participants represented a range of age groups and demographic backgrounds. The participants were then divided into two groups: CoronaVac Recipients and ChAdOx1 Recipients. The selection of participants into each group was based on the vaccine availability and policy of MoH and local government [24].
The author passively observes the selection with no decisive role in the process of participant screening. The study design was explained and discussed fairly with the allocated participants before informed consents were provided and enrolled as the subject of the study. Participants without formal informed consent were not enrolled as subjects of the study.
For the two groups, two doses of the CoronaVac and ChAdOx1 vaccines were administered respectively according to the recommended schedule by the Ministry of Health of Indonesia [22]. Initially, the study was designed to monitor the antibody level during 18 months after second dose administration. Antibody levels were measured just before the first dose (time-point or TP1), two weeks after the first dose (TP2), just before the second dose (TP3), 1 month after the second dose (TP4), 12 months after the second dose (TP5), and 18 months (TP6) after the second dose (Fig. 1).
Meanwhile, during 6-12 months after the second dose, the MoH released a policy to implement booster administration [25]. The subjects were then administered with a third dose based on vaccine availability and local government policy. The third dose that was administered was ChAdOx1 as a homologous booster (for the previous two doses of ChAdOx1 recipients) as well as a heterologous booster (for the previous two doses of CoronaVac recipients). The antibody levels in the 12 months and 18 months after the second dose were measured as initially designed.
The total sampling method was implemented based on the number of participants on the site during the cohort period. Lost follow-ups were anticipated during the 18 months, and coordination with stakeholders and intensive preparation were implemented intensively to minimize the number. This study focused on the subjects that followed all the designated TP and were administered with ChAdOx1 as the third dose before antibody level measurement on TP5 to analyze the antibody level among homologous and heterologous booster groups.
2.2. Data Collection
A blood sample was drawn from the subject on every designed time-point (TP1-TP6). A blood sample was taken using conventional sampling techniques. The CMIA method was used to measure the antibody response. Accredited clinical laboratories measure by the instrument's use guidelines. The SARS-CoV-2 IgG II quantitative assay technique was used for antibody testing Chemiluminescent microparticle immunoassay (CMIA) technology was used to quantitatively and qualitatively identify IgG antibodies to SARS-CoV-2. Reactivity to COVID-19 antibody was set on the cut-off of 50 AU/mL as declared by the guideline of the kit [26].
Along with the blood sample collection, sex, age, and BMI were collected to determine the subjects’ characteristics. Beyond that, the study recognized that scheduling of vaccination, economic aspects and the rise and down of COVID-19 cases, were among the uncontrolled variables influencing the subject during the cohort study. Microsoft Excel packages and SPSS v25.0 software (SPSS Inc., Chicago, IL) were used for data analysis. The data of subjects were verified to be valid in all TP1 to TP6 for analysis. For any missing data, the related subject) data were excluded from the analysis. An independent t-test or Wilcoxon test was used to compare antibody responses between the two groups after the third vaccination dose.
The time-point during 18 months cohort after the second dose administration.
All the data supporting the findings of the article are available within the article. Data were analyzed as groups of CoronaVac and ChAdOx1 recipients, between with or without antibody reactivity on TP1, and based on the characteristics of sex, age groups, and BMI.
3. RESULTS
A total of 153 subjects completed the study period, comprising 87 subjects in the ChAdOx1 group and 66 subjects in the CoronaVac groups, who were enrolled and actively participated during 18 months of the cohort. Those two types of vaccines were among the dominant types used in the Indonesian COVID-19 Vaccination Program.
The serum samples of each subject were collected from TP1 through TP6. On TP1, serum samples were drawn just before the first dose administration. Table 1 shows the characteristics of subjects and the number of subjects with or without positive reactivity to COVID-19 Antibody on TP1 (baseline data).
Most of the subjects were female (64.05%) in two groups. The dominant number of females was not by design and was not parallel with the proportion of male and female citizens of Indonesia. It was related to vaccine availability and local government policy. Most subjects were aged between 36 and 59 years (72,55%), with an average age of 41.91 years. The age grouping was based on the guidelines of the Indonesian MoH [27]. Also, based on body mass index (BMI), the majority of the subjects showed normal BMI (47.06%) and were overweight (28,76%).
There were 66 subjects in the CoronaVac group, with 3 of them being>60 years old. Considering the small number, then the following analysis excluded these subjects and only included the 63 subjects. The total number of subjects added to the ChAdOx1 group was 150.
On TP1, immediately before the first dose administration, a blood sample was taken. COVID-19 antibody reactivity was detected in 100 (66.67%) of the subjects. The relatively high number indicated the high prevalence of asymptomatic COVID-19 infection, mainly during the highest toll in June-August 2021.
The data of single or multiple sessions of previous infection were out of the study coverage. Among the two groups, the difference proportion of subjects with COVID-19 antibody reactivity was not by design since the occurrence was random, while the division into groups was based on vaccine availability and local government policy.
The BMI showed no simple conclusion to draw on the relationship with COVID-19 antibody reactivity. Even in the subjects with normal BMI, the proportion of previous infection was high, as in the overweight subjects. In contrast, it was relatively low in the subjects who were underweight or obese.
Overall, regarding age groups and BMI, the study did not document a significant differentiation antibody level between CoronaVac and ChAdOx1 groups during TP1 to TP6 (p>0,05 in all measured variables, Table 1).
Fig. (2) shows the kinetic of COVID-19 antibody levels during the cohort of TP1 through TP6 as designed in detail in the method section. These kinetics were independent of (part A) or based on the antibody reactivity status on TP1 (part B).
| Variables | CoronaVac | ChAdOx1 | N | % | p | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Reactive* | Not Reactive* | N | Reactive* | Not Reactive* | ||||||||
| N | % | N | % | N | % | N | % | ||||||
| Sex | >0.05 | ||||||||||||
| Male | 18 | 7 | 10.61 | 11 | 16.67 | 34 | 27 | 31.03 | 7 | 8.05 | 52 | 33.99 | |
| Female | 45 | 17 | 25.76 | 28 | 42.42 | 53 | 49 | 56.32 | 4 | 4.60 | 98 | 64.05 | |
| Total | 63 | 24 | 36.36 | 39 | 59.09 | 87 | 76 | 87.36 | 11 | 12.64 | 150 | ||
| Age group (years) | >0.05 | ||||||||||||
| 18-35 | 20 | 9 | 38.09 | 11 | 61.91 | 19 | 13 | 87.36 | 6 | 12.64 | 39 | 25.49 | |
| 36-59 | 43 | 15 | 34.88 | 28 | 65.12 | 68 | 63 | 92.65 | 5 | 7.35 | 111 | 72.55 | |
| 60+ | 3** | ||||||||||||
| Body Mass Index | >0.05 | ||||||||||||
| Underweight | 3 | 1 | 1.52 | 2 | 3.03 | 6 | 4 | 4.60 | 2 | 2.30 | 9 | 5.88 | |
| Normal weight | 33 | 15 | 22.73 | 18 | 27.27 | 39 | 34 | 39.08 | 5 | 5.75 | 72 | 47.06 | |
| Overweight | 17 | 6 | 9.09 | 11 | 16.67 | 27 | 26 | 29.89 | 1 | 1.15 | 44 | 28.76 | |
| Obesity | 10 | 2 | 3.03 | 8 | 12.12 | 15 | 12 | 13.79 | 3 | 3.45 | 25 | 16.34 | |
** As too small number of elderly subjects, the data are excluded from the following analysis.
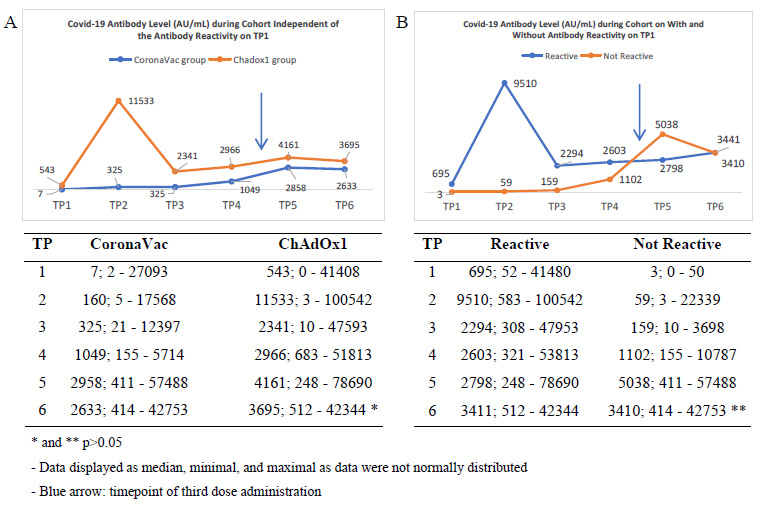
Kinetics of COVID-19 antibody level during cohort (part A) independently from the reactivity and (part B) based on the reactivity status on TP1.
Of note, considering the mean and standard deviation of antibody level, it was indicated that the response varied broadly among the subjects. Statistical analysis showed a non-normal distribution of data. The results and analysis were then displayed as median and minimal-maximal values.
On TP2, two weeks after the first dose, subjects receiving ChAdOx1 averagely showed a high increase in antibody level that was very significantly different from the CoronaVac group. It is worth noting that, the median, minimal, and maximal values, even on TP2, had an overlap of antibody levels between groups. It indicated the variety of humoral responses to vaccines that might be related to other factors.
The rise was followed by a sharp decrease in TP3 just before the second dose was administered, which was still significantly different but in a narrower gap with the average level in the CoronaVac group. On TP4, one month after the second dose administration, there was just a slight increase in the two groups while keeping the narrow gap between both. During the time between TP4 and TP5, a portion of subjects were administered with the third dose. The types of which were in variety. The study focuses on the portion of the subject that was being administered with ChAdOx1 as the third dose.
On TP5, there was a rise in the antibody level of two groups, with the gap narrowing. Eventually, on the TP6, the level slowly decreased with the gap was more narrowing and showed no significant difference between the two groups.
Considering the reactivity status of TP1, in Fig. (2) Part B, there was a high increase in TP2 among the reactive groups. It was then followed by a sharp decrease and a narrowing gap with the antibody level of the group that was not reactive. The interesting trend was on the TP5 that after being administered with the booster, the group that was not reactive showed an increase even more than the antibody level of the reactive group. It was suspected as a temporary reaction since the levels were becoming very close on TP6, and no significant differences were documented.
4. DISCUSSION
Early reports of patients with cough, lung ground glass opacities, and symptoms progressing to severe pneumonia suggested that SARS-CoV-2 could be transmitted through the respiratory system. Direct transmission by respiratory droplets is confirmed by productive SARS-CoV-2 replication in both the upper and lower respiratory tracts (LRT), as well as the rising number of cases demonstrating human-to-human transfer among close contacts exhibiting vigorous coughing [28].
Accurate virus detection through RT-PCR is a first step toward containing the COVID-19 pandemic. Lapses jeopardize public safety by allowing illness to spread, helped by misleading negative test findings. Serological testing complements virus detection by indicating prior infection, which could be used for therapeutic gain [29]. Dealing with the risk, during the examination of a suspected COVID-19-infected patient, a modification was made to minimize the risk of transmission as well as radiation exposure [30-32].
Given the burden of containing the pandemic, the reasonably successful preventive method was to keep both physical and social isolation. Even in the past, artificial intelligence has proven to be a promising solution to everyday life problems. The required prototype will be provided with some instructions on using Python, CV, and deep learning to keep track of people and their distance [33].
As distancing was a temporary strategy, numerous vaccine platforms have been developed to elicit a potent immune response and protect people from severe COVID-19. Vaccination is one of the most important methods of stopping the spread of the virus and protecting the general public from the harmful effects of COVID-19 infection [34, 35]. Even recently, there was still peoples’ mistrust in the COVID-19 vaccine monitored through social media [36].
CoronaVac is a vaccine that is currently widely used in several countries, and it works by stimulating the immune system with an inactivated virus. The preliminary study suggests that CoronaVac vaccination significantly reduces the risk of contracting COVID-19, becoming seriously ill, and dying from the virus. However, other studies have shown that the level of protection varies depending on the vaccination and demographic context. Studies in many countries show that effectiveness rates in preventing serious illness or death vary from fifty percent to more than eighty percent [37-39].
Meanwhile, ChAdOx1, which uses a modified adenovirus vector to deliver the viral spike protein gene into the body, is one of the most widely used vaccines in the world today. Clinical trials suggest that the ChAdOx1 vaccine is reasonably effective in reducing the risk of severe illness, hospitalization, and mortality associated with COVID-19. Concerns have been raised in some countries about the extremely rare possibility of blood clots forming following this vaccination, although the risk is very low [40, 41].
Booster doses can be administered using the same type of vaccine as the previous dose (homologous booster) or a different type of vaccine (heterologous booster). Population studies in Northern Cyprus, Turkey, have shown that administration of a third single booster dose of both Pfizer and CoronaVac vaccines can increase the level of anti-spike RBD IgG antibodies in elderly populations. In this study, a booster dose was administered to recipients of a complete two-dose CoronaVac/CoronaVac vaccine regimen. Although both vaccines increased antibody levels, subjects who received a Pfizer booster (a heterologous booster) had an 8-fold increase in antibody levels [42].
A study conducted in Thailand compared groups receiving only two doses of CoronaVac with those receiving an additional dose of AstraZeneca (a heterologous booster). The results of the study showed a more than 80-fold increase in antibody level in the group receiving an AstraZeneca booster (6.763 BAU/mL; 4.626-11.525 BAU/mL; p < 0.0001) compared to the group receiving only one dose of CoronaVac (60.97 BAU (binding antibody unit)/mL, 47.95-127.9 BAU)/mL). The study also found that the group that received an additional dose of Pfizer (21.457 BAU/ml; 15.571-34.266 BAU/mL; p<0.0001) also had an increase in antibody level that reached more than 200 times that of the group that did not receive an additional dose [43].
A study conducted in Botswana evaluated the immune response following administration of AstraZeneca's vaccine in the 1 dose, 2 dose, and booster groups. The results of the study showed that the group given a booster dose had anti-SARS CoV-2 level, remained awake, and increased from 28.4 GMC (95% CI 14.1-57.4) to 35.3 GMC (95% IC 23.2-53.6) on day 182 after dose 1 compared to before the booster dose (day 170 after the first AZ dose). The limitation of this study was the lack of data on antibody levels in a group that received only two doses of the vaccine on days 170 and 182 after the first dose. However, on these two days, a small proportion of the population in the two-dose group was still found to be seropositive [44].
The study showed that the kinetics of average COVID-19 antibody levels among the subjects were dynamically different between the CoronaVac and ChAdOx1 groups. Independent of antibody reactivity status, the difference in the kinetics was very significant for TP2. This is in coherence with the understanding that there were different responses between types of COVID-19 vaccine, mainly between inactivated whole-virus and viral vector vaccines, as also reported previously [45].
The rise in TP2 was followed by a decrease that was sharp in ChAdOx1 groups and slight in CoronaVac groups. This is in coherence with the understanding of short response after the first dose, that the immune system was well-informed for a long effect after the second dose administration on TP3. The slight increase towards TP4 indicated a gradual increase of antibody levels toward long-term effect as targeted with vaccination as a reflection of long-term IgG antibody product [46].
A slight increase was also noted after the third dose of ChAdOx1, with the gap narrowing between the two groups. It was noted that even on the previous recipients’ CoronaVac subject, the increase was like that previously being administered with ChAdOx1. It indicated that there was no significant difference between homologous and heterologous boosters. The relatively mature IgG was expected to respond similarly in the two groups upon homologous and heterologous boosters. It was even more strongly shown on TP6 that the gap was narrowing and became not significantly different [47].
It was interesting that the antibody level of the reactive group steadily increased from TP3 to TP6, indicating that a longer period from the previous infection led to a maturity of humoral response than the non-reactive group. Nevertheless, the most important point is that homologous and heterologous provide no different antibody levels during an 18-month cohort.
In addition to the antibody response, the effect of the third dose of the ChAdOx1 vaccine on defense against COVID-19 infection and disease should also be considered. Effective protection involves not only the antibody response but also the cellular immune response and other elements. The antibody response is an important signal of adaptive immunity. In populations with heterologous boosters, further studies with long-term follow-up are needed to assess the efficacy of the third dose of the ChAdOx1 vaccine in preventing severe COVID-19 disease and SARS-CoV-2 infection [40, 48].
Other research suggests that the type of previous immunization may have an impact on the immunological response to a third dose of the COVID-19 vaccine [49, 50]. Furthermore, response to the third dosage of the ChAdOx1 vaccine might be affected by age, gender, and prior immunization status. While elderly people or those with deteriorating health conditions may have fewer antibody responses, younger people often show stronger antibody responses. These results bolster the significance of taking immunological and demographic variables into account when designing COVID-19 immunization programs, particularly when multiple doses are involved [40, 50].
The study documented no significant difference among the subjects based on sex, age, and BMI. Among the possible explanations, the subjects were enrolled based on the policy of sites and the availability of vaccines on the sites. It is one of the drawbacks of the study. The broader scale of study on a more varied number of sites and randomly selected subjects is necessary to adjust and analyze the effect more properly.
The inactivated whole-virus type of vaccine (CoronaVac) was most frequently used in Indonesia’s COVID-19 vaccination program during the pandemic. The viral vector vaccine (ChAdOx1) was the second. The study indicated that both vaccines showed different kinetics during a short period but eventually induced no significant difference in antibody response in the cohort until 18 months. It is imperative that nowadays, the product of the inactivated whole virus vaccine is regulated to be dominantly administered during the post-pandemic COVID-19 vaccination in Indonesia [51, 52].
Consideration of vaccination policy and defense strategies during the transition period requires consideration of economic, logistical, and etiological factors. The dosage and distribution of vaccines must take into account the most urgent needs of the population and the availability of daily supplies for COVID-19. It is also important to consider ethical guidelines for resource distribution and ease of access to ensure that public sector efforts reach each segment of the community equitably [53].
The type of vaccine was based on local availability and government policy. Those were the two dominant types being used in the Indonesian COVID-19 Vaccination Program. The study was not authorized to analyze other type of vaccines. The participants’ selection was also based on government policy. Overall, the above-mentioned were the limitations of the study.
It is also a drawback that the study only d had a very small number of elderly people, which renders it difficult to conduct a proper analysis. However, for the general population, the results showed no significant difference between homologous and heterologous groups at 12 months after the third dose and 18 months after the second dose. It widens the opportunity to have a booster vaccine without constraint on the same type of vaccine availability.
It is also imperative to note that the level of antibodies does not merely reflect the protection level of the COVID-19 infection. The neutralizing antibody should be measured to more precisely indicate the protection level. Further study was necessary to elucidate the neutralizing level of the reported antibody level to more precisely reflect the protective level of COVID-19 infection.
CONCLUSION
Overall, this study has provided information on the immunological response to the COVID-19 vaccine, particularly concerning homologous and heterologous boosters. The information should be important to build, maintain, and strengthen the strategy to prepare the public for the possible threat of COVID-19. Further study is necessary to elucidate deeper information on humoral immunity protection levels against infection as well as severe illness and death caused by COVID-19.
AUTHOR’S CONTRIBUTIONS
It is hereby acknowledged that all authors have accepted responsibility for the manuscript's content and consented to its submission. They have meticulously reviewed all results and unanimously approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| MoH | = Ministry of Health |
| CMIA | = Chemiluminescent Microparticle Immunoassay |
| BMI | = Body Mass Index |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Research Ethical approval was granted by the Ethics Committee of the National Institute of Health Research and Development, Ministry of Health, Indonesia (MoH No.: LB.02.01/2/KE. 431/2021).
HUMAN AND ANIMAL RIGHTS
All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committees and with the 1975 Declaration of Helsinki, as revised in 2013.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article is available in the Zenodo at https://zenodo.org, reference number DOI 10.5281/zenodo.13137371.
FUNDING
The study is partially supported by the grant of Sebelas Maret University, with the ID of Research Group Scheme 00070574054502024, No of Grant 194.2/UN27. 22/PT.01.03/2024.
ACKNOWLEDGEMENTS
Thanks to The National Institute of Health Research and Development, Ministry of Health, National Research and Innovation Agency, Provincial and City/Regency Health Services, and Primary Health Care. Also, we are grateful to the Universitas Sebelas Maret (UNS) Institute for Research and Community Service (LPPM).


