All published articles of this journal are available on ScienceDirect.
Comparing the Effect of Chamomile and Mefenamic Acid on Primary Dysmenorrhea Symptoms and Menstrual Bleeding: A Randomized Clinical Trial
Abstract
Background:
Dysmenorrhea in young women reduces their quality of life.
Objectives:
This research reviewed the impact of chamomile sachet and mefenamic acid on primary dysmenorrhea, its relevant symptoms as well as bleeding.
Methods:
Two hundred female students afflicted with primary dysmenorrhea from Arak universities were randomly assigned to two groups and participated in this randomized clinical trial. The first group (A) received mefenamic acid (250 mg) and the second group (B) received chamomile (5000 mg) three times a day in two consecutive cycles from two days before up to the first three days after menstruation. Intensity of pain, related symptoms and bleeding were evaluated by visual analog scale, Andersch-Milsom Verbal Scale and Higham chart, respectively. Data analysis was performed by SPSS 21.
Results:
Severe pain lasting two months after intervention was observed in 6 subjects (6.3%) of group (B) as well as 6 participants (6.3%) in group (A) (p=0.351, p=0.332). Two months after treatment, mean severity of related symptoms was 4.93±3.54 in group (B) and 5.62±3.54 in group (A), which shows further reduction of pain in group (B) that was not significant (p=0.278). Two months later, mean of bleeding was 88.71±66.4 and 70.54 ±53. 34 in group (B) and (A), respectively. Thus, the decrease of pain in the two groups was not significant (p=0.567).
Conclusion:
It appears that chamomile sachet can decrease the severity of pain and bleeding, which is similar to the effect of mefenamic acid and even further alleviates the symptoms of dysmenorrhea.
(IRCT code no. 20161008250B1N5).
1. INTRODUCTION
Primary dysmenorrhea is among the most frequent problems for young women, which is accompanied with painful menstruation without a known pathologic cause that begins with crampy abdominal pain a short while before menstruation lasting up to 72 hours. Nevertheless, the highest intensity of pain is observed within the first 48 hours of menstruation given the rising secretion of prostaglandin over this period [1]. Different degrees of pain (60-90%) have been reported in studies conducted concerning the prevalence of primary dysmenorrhea in young women [2]; however, the pain in a number of patients is so severe that it interferes with their daily activities and is a main factor of absenteeism both from school and work [3, 4].
Initial dysmenorrhea appears to be a function of PGF2α overproduction or rising ratio of PGF2α to PGE2, which is attested with the detection of high E2 and F2α levels in endometrium of women with primary dysmenorrhea. Prostaglandins can lead to severe uterine contractions, as well as fatigue, dizziness, headache, nervous change and related symptoms [5-7]. Several treatments have been used for primary dysmenorrhea and its associated symptoms, including contraceptive pills [8, 9], vitamin E compounds [10], vitamin D [10-12], fish oil supplements [13], diet [14], exercise and physical activity [15], acupuncture [16], topical heat [17], psychotherapy [18] and NSAIDs.
NSAIDs have a prominent role in the suppression of inflammation and decrease prostaglandin levels [19]. These drugs significantly decrease PG concentration of endometrial fluid and reduce uterine tone by inhibiting cyclooxygenase enzyme. Mefenamic acid is a common NSAID causing a variety of adverse effects like diarrhea, stomachache, and nausea to serious conditions such as chronic kidney disease and osteoporosis [20]. Today, complications of chemical drugs have shifted the attention of researchers toward complementary therapies [21, 22].
Chamomile plants, including Chamomilla recutita and Matricaria chamomilla, exert positive effects on dysmenorrhea due to their anti-inflammatory activity [23]. Chamomile is effective in reducing abdominal and pelvic pain, fatigue, lethargy and depression during menstrual cycles [24]. The ethanolic extract derived from the flowers of this plant has anti-spasmodic, anti-inflammatory, anti-anxiety and sedative properties [25]. Researchers have recommended further studies regarding the side effects and benefits of complementary medicine, although US Food and Drug Administration states that chamomile has no side effects on pregnancy, lactation or children [26, 27]. However, researchers have reported no side effects in chamomile use except for skin dermatitis in case of allergy to it as well as sore throat, and its consumption is recommended for all people except for breastfeeding and pregnant women [28-30]. Also, in rare cases, chamomile infusion has been associated with anaphylactic reactions and its presence in eyewashes can cause allergic conjunctivitis [31-32].
Several studies have been conducted regarding the efficacy of chamomile as well as mefenamic acid, which indicate that chamomile is as effective as mefenamic acid in alleviating pain; however, the impact of chamomile on menstrual bleeding has been different. Also, there have been few studies regarding symptoms related with dysmenorrhea [33, 34]. Consequently, there is a need for further studies using different forms and doses of chamomile and recruiting a larger number of subjects who live in various places to detect new results of chamomile effects on dysmenorrhea and its related symptoms [22, 34-40].
This research was conducted to compare the effect of chamomile sachet with mefenamic acid capsule on primary dysmenorrhea, its related symptoms and the volume of menstrual bleeding.
2. METHODS
2.1. Study Design
The present study, which is a double blind randomized clinical trial, was conducted on 200 female students from four universities of Arak in 2018. There are six universities in Arak, and sampling was performed by drawing from four universities.
2.2. Study Participants
Two hundred female students meeting the following inclusion criteria participated in this study. Single students having moderate to severe menstruation pain (≥4 pain intensity based on visual analog scale), an age range of 18-30 years, menstruation pain started before 20 years of age, regular menstruation cycles with intervals of 21-35 days, menstrual bleeding lacking passing clots (low and moderate bleeding), onset and duration of menstruation from a few hours before menstruation up to day 5 of bleeding. Other criteria included the absence of chronic diseases, no consumption of drugs such as anticoagulants, oral contraceptives, narcotics, benzodiazepines, lack of regular exercise or abnormal vaginal discharge, history of allergy to herbs, absence of stressors in the last two months, specific dietary requirements, history of pelvic inflammatory disease, myoma and pelvic tumors, record of gynecological surgery, transfer to another university and graduation from the university within the next six months.
The exclusion criteria from this research were as follows: the decision of participant to leave the study, having any disease requiring prolonged or continuous medication, dietary supplements and vitamins, reluctance to consume the drug for any reason, failure to complete the pain severity table and other questionnaire items, incidence of stressful conditions in the past two months, history of contraceptive use, undergoing any surgery during the study, getting married over the study period, changing the residence and not taking the drugs according to the protocol.
2.3. Sample Size
According to calculations, 45 students were assigned to each group based on confidence interval of 95%, study power of 80% as well as minimum average difference score (d) of 0.5. Given the insufficient quantity of drugs and other consumables, the number of samples reached 90, and 100 participants were placed in each group after adding a possible dropout rate of 10%.
We used the following formula to calculate the sample size:
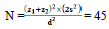 |
2.4. Study Procedure
After approval by Ethics Committee of Arak University and receiving IRCT code as well as an authorization letter from Arak University of Medical Sciences, the researcher visited the universities for sampling. Announcements were made to recruit students with menstrual pain. The researcher explained the study objective and its implementation method to the students after visiting them. A checklist including demographic information, as well as midwifery, inclusion and exclusion requirements was submitted to the subjects who were instructed to fill out the checklist, especially the pain scale. After completion of the checklist, the eligible students were randomly invited to collaborate in the research according to sample size.
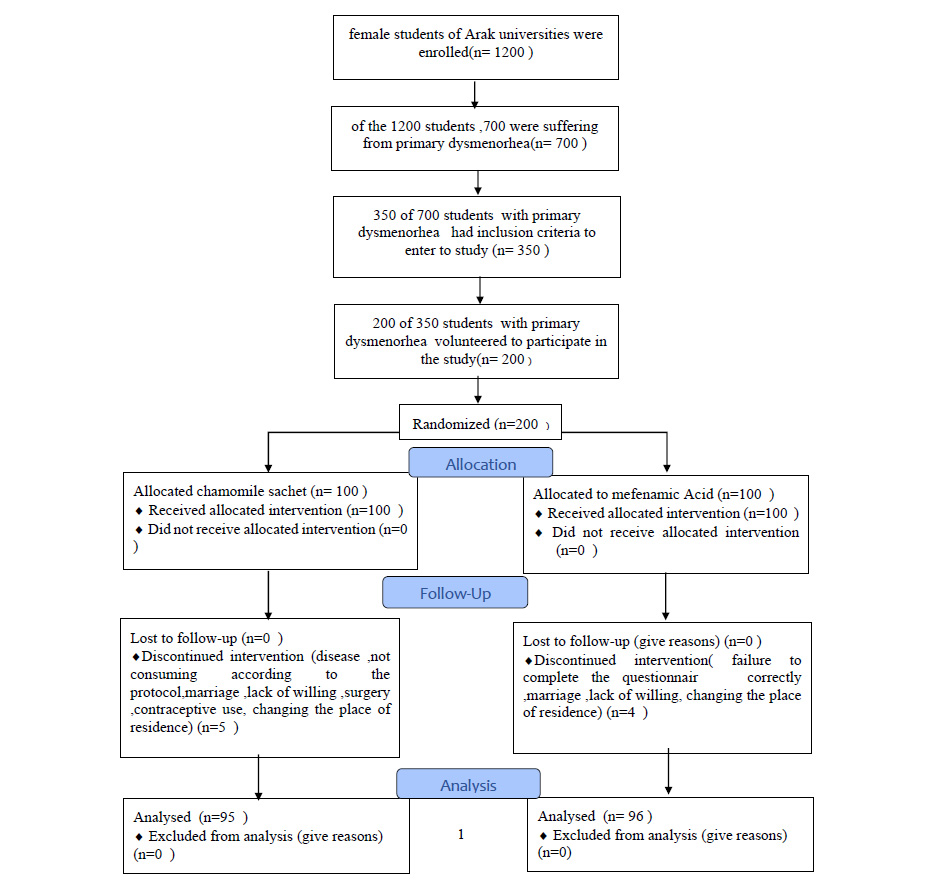
From a total of 1200 participants eligible to enter the project who were willing to cooperate, 700 were suffering from primary dysmenorrhea, and 350 students with primary dysmenorrhea met the inclusion criteria. Finally, 200 out of 350 students with primary dysmenorrhea volunteered to participate in the study after presenting written informed consent forms. Using random blocks, the subjects were randomly assigned to two groups: mefenamic acid (group A) and chamomile sachet (group B). The contents were wrapped in envelopes of the same shape and then the blinding was done. Also, the statistician was completely unaware of the allocation of groups (double-blind). The willingness of the company to conduct research and maintain the information confidential was emphasized for research units.
The researcher repeatedly described the research procedure to the subjects, and there was an emphasis on timely consumption of drug and correct filling of questionnaire, especially measurement of pain intensity, completion of bleeding chart and determining the severity of symptoms. Moreover, the address and phone number of researcher was given to students for follow-up.
To observe the ethical considerations for pain relief, the volunteers could choose another residence if they did not control their pain and were obliged to record the exact type of dwelling.
For each group, drugs as well as sanitary pads with medium size purchased from the same brand and lacking absorbent material were given to students to measure the amount of menstrual bleeding in pre-intervention cycle as well as two consecutive interventional cycles.
The sachet of chamomile included 5000 mg of chamomile, and a spoonful of honey was also consumed per serving. The honey was added in small amounts only as a flavoring with sachet of chamomile to improve its bitter taste. Studies have indicated that therapeutic doses of honey can be effective for treatment of dysmenorrhea [41, 42]. The sachet contents were gently boiled in a glass of water for three minutes, were filtered and dissolved in a spoonful of honey and subsequently ingested [33].
On the other hand, 250 mg capsules of mefenamic acid were consumed by subjects in mefenamic acid group. The two groups received the same instructions two days before menstruation up to three days after it, and three daily servings were given for two consecutive cycles. The researcher monitored timely consumption of medicinal plant and mefenamic acid and was also in contact with students in cyberspace. In this study, five subjects in chamomile sachet and four in mefenamic acid group did not continue their participation in the study (Fig. 1).
2.5. Data Collection Tools
Data collection was done using a questionnaire that was filled in three times. Questionnaires 1, 2 and 3 were completed by research units before intervention, as well as one and two months after it. The questionnaire included demographic information, pain intensity and duration, measurement using pain ruler; menstrual blood loss was evaluated using a pictorial chart and the symptoms of dysmenorrhea were evaluated by Andersch-Milsom verbal scale.
The degree of dysmenorrhea pain was assessed using Visual Analog Scale (VAS), with 8-10, 4-7, and 1-3 scores representing severe, moderate, and mild pain, respectively. We used Pictorial Bleeding Assessment Loss Chart (PBAC) to measure the extent of bleeding, which indicates an estimate of blood loss through menstruation on a sanitary pad with mild (score 1), moderate (score 5), and severe (score 20) criteria. In this chart, a small stain of blood on sanitary pads receives score 1, large stains score 5, and impregnation with blood score 20. The chart also rates small and large clots. Small clots score 1, large ones 5, and flow of blood 10. A score <50 is indicative of mild bleeding, 51-100 moderate bleeding, and >100 hypermenorrhea during menstruation. The scores were added up to calculate the overall score. The symptoms associated with dysmenorrhea were assessed based on Andersch-Milsom Verbal Multidimensional Scoring System. Four grades have been cited in this scale for the intensity of dysmenorrhea symptoms as follows. Score 0 (no symptoms); score 1 (mild symptoms present but not interfering with daily activities); score 2 (symptoms exist interfering with daily tasks but are moderate and not debilitating); score 3 (symptoms quite debilitating and severe) [15].
2.6. Isolation of Essential Oil
In a Clevenger type apparatus, 500 g of chamomile flower was hydro distilled for 12 h to isolate the essential oils, which were subsequently kept in dark conditions at -4°C for GC/Mass analyses.
Gas chromatography–mass spectrometry was performed by Perkin-Elmer autosystem XL GC that was equipped with ZB- 5 capillary column (5% phenyl–95% dimethyl polysiloxane, 30 mm × 0.25 mm × 0.25 µm). The mobile phase was helium with a flow rate of 1 ml/min.
3. RESULTS
3.1. Composition of Essential Oil
α-Bisabolol oxide was found to be the main constituent (34%) as indicated by GC/MS analysis in chamomile oil, followed by chamazulene (12%), 1,8-cineole (8%), and limonene (5%) as the main compounds.
3.2. Demographic Characteristics
The demographic and midwifery features of subjects were evaluated by Kruskal-Wallis test (age, menarche, age of dysmenorrhea onset), Fisher’s exact test and chi-square test (education level, menstruation cycle, length of menstruation bleeding per day, and regular menstruation) as shown in Table 1.
3.3. Symptoms of Dysmenorrhea
Friedman test results revealed a significant decrease in the severity of pain one and two months after treatment in group A (p=0.001) as well as in group B (p=0.001), and Fisher’s exact test indicated that the frequency distribution of pain intensity within one (p=0.351) and two months (p=0.332) of treatment was not significantly different between the two groups (P=0.001) (Table 2).
The results of within group repeated measures ANOVA test showed that mean score of pain days (p<0.05), mean Higham score (p=0.0001, F=20.02), mean changes in associated symptoms of dysmenorrhea (p = 0.005, F= 5.47) had significant difference between groups, and paired comparisons Bonferroni test indicated that changes in scores of symptoms in both groups were significant up to one month following the intervention (Table 3).
Results of between-group repeated measure test with control for confounding effects (i.e. age of dysmenorrhea occurrence) showed no significant difference in mean score of pain days (p = 0.248, F = 1.371), mean score of related symptoms (p = 0.278, F = 1.183) and score of Hingham with confounding effect (Hingham score in baseline and age of dysmenorrhea occurrence) between the two groups (p = 0.567, F = 0.329) (Table 3).
| Mefenamic Acid N(%) |
Chamomile N(%) | P-value | ||
|---|---|---|---|---|
| Education level | Associate degree | 9(9.4) | 4. (4.2) | 0.106 |
| Bachelor’s degree | 82(85.4) | 83 (87.5) | ||
| Master’s degree | 1(1) | 6 (7.3) | ||
| PhD | 3(3.2) | 2(1) | ||
| GP | 1(1) | 0 | ||
| The length of menstruation bleeding (day) | 3 | 3(3.1) | 3(3.1) | 0.114 |
| 4-7 | 78(81.3) | 86(90/6) | ||
| 7 | 15(15.6) | 6(6.3) | ||
| Regular menstruation | Yes | 82(85.4) | 76(80.2) | 0.329 |
| No | 14(14.6) | 19(19.8) | ||
| Menstruation Cycle | 21 | 12(12.5) | 11(11.6) | 0.593 |
| 22-35 | 83(86.5) | 84(88.4) | ||
| >35 | 1(1) | 0 | ||
| Mean | Mean(23) | |||
| Age (Mean±Standard deviation) |
20.88(1.74) | 21.5(2.24) | 0.145 | |
| Menarche (Mean±Standard deviation) |
13.1(1.48) | 13.35(1.3) | 0.323 | |
| Beginning age of Dysmenorrhea (Mean±Standard deviation) |
15.46(2.36) | 15.05(2.18) | 0.258 |
Frequency distribution using Fisher’s exact test and chi-square test (Level of education, Menstruation Cycle, length of menstruation bleeding (day), Regular menstruation).
| Intervention | Pain Severity | Before Intervention | One Month Later | Two Month Later | P value (Friedman) |
|---|---|---|---|---|---|
| N (%) |
N (%) |
N (%) |
|||
| Mefenamic acid | 0 | 0 | 3(3.2) | 4(4.2) | 0.001 |
| 1-3 | 0 | 44(45.8) | 51(53) | ||
| 4-7 | 50(52.1) | 39(40.6) | 35(36.5) | ||
| 8-10 | 46 (47.9) | 10(10.4) | 6(6.3) | ||
| Chamomile | 0 | 0 | 8(8.3) | 10(10.4) | 0.001 |
| 1-3 | 0 | 36 (37.5) | 44(45.8) | ||
| 4-7 | 44(45.8) | 43(44.8) | 36(37.5) | ||
| 8-10 | 52(54.2) | 9(9.4) | 6(6.3) | ||
| 0.470 | 0.351 | 0.332 | P value (Fisher) |
||
Table 3.
| Variable | Group |
Baseline Mean±SD |
One Month Later Mean±SD |
Two Month Later Mean±SD |
Repeated Measure Test (Pairwise Comparisons Bonferroni) |
Repeated Measure Test (Between Group) | ||
|---|---|---|---|---|---|---|---|---|
| 0-1 | 0-2 | 1-2 | ||||||
| Pain days in dysmenorrhea | Chamomile | 2.43±0.98 | 1.86±0.7 | 1.82±0.57 | 0.0001 | 0.0001 | 0.310 | F=1.371 p-value= 0.248 |
| Mefenamic acid | 2.31±0.98 | 1.79±0.8 | 1.70±0.73 | 0.005 | 0.0001 | 0.165 | ||
| P-value | 0.605 | 0.510 | 0.3 | |||||
| Symptoms of dysmenorrhea | Chamomile | 7.22±3.78 | 5.41±3.98 | 4.93±3.54 | 0.0001 | 0.0001 | 0.127 | F=1.183 p-value= 0.278 |
| Mefenamic acid | 7.62±3.97 | 6.18±3.8 | 5.62±3.54 | 0.0001 | 0.0001 | 0.05 | ||
| P-value | 0.464 | 0.108 | 0.162 | |||||
|
Higham (PBAC) |
Chamomile | 101.85±67.38 | 89.72±63.61 | 88.71±66.4 | 0.119 | 0.0001 | 0.312 | F=0.329 p-value= 0.567 |
| Mefenamic acid | 109.06±72.84 | 82.49±58.89 | 70.54±53.34 | 0.291 | 0.325 | 0.297 | ||
| P-value | 0.743 | 0.263 | 0.131 | |||||
Mean of pain days: within-group (repeated measures within-group test)..
Mean value of associated symptoms of dysmenorrhea: between groups (between group repeated measure test).
Mean value of associated symptoms of dysmenorrhea: within-group (repeated measures within-group test, followed by Bonferroni test)..
Mean Hingham scores: between groups (between group repeated measure test).
Mean Hingham scores: within-group (repeated measures within-group test, followed by Bonferroni test).
Comparison of each of symptoms in detail by Friedman test showed that although the mean severity of symptoms in chamomile sachet group was further reduced relative to mefenamic acid group, no significant difference was found in this regard one and two months after the treatment between groups (p=0.262 and p=0.131, respectively). Symptoms such as back pain, headache, dizziness, diarrhea, fatigue and anorexia in chamomile sachet group showed a considerable reduction in average intensity (p = 0.001). Besides, the mean severity of symptoms like vomiting, fainting and mood changes were remarkably decreased in mefenamic acid group but was not significant (Table 4).
| - | - | Chamomile | Mefenamic Acid | - |
|---|---|---|---|---|
| Back pain | Before the intervention | 1.91±1.01 | 1.86±0.88 | 0.563 |
| One month later | 1.218±0.92 | 1.28±0.84 | 0.802 | |
| Two month later | 1.08±0.91 | 1.17±0.8 | 0.479 | |
| 0.001 | 0.001 | |||
| Vomiting | Before the intervention | 0.364±0.727 | 0.531±0.983 | 0.508 |
| One month later | 0.250±0.68 | 0.343±0.83 | 0.515 | |
| Two month later | 0.187±0.62 | 0.229±0.73 | 0.873 | |
| 0.006 | 0.001 | |||
| Diarrhea | Before the intervention | 0.531±0.75 | 0.281±0.67 | 0.02 |
| One month later | 0.437±0.85 | 0.208±0.52 | 0.074 | |
| Two month later | 0.333±0.57 | 0.25±0.63 | 0.057 | |
| 0.001 | 0.487 | |||
| Dizziness | Before the intervention | 0.468±0.69 | 0.572±0.86 | 0.726 |
| One month later | 0.281±0.59 | 0.479±0.78 | 0.044 | |
| Two month later | 0.25±0.56 | 0.416±0.73 | 0.094 | |
| 0.001 | 0.019 | |||
| Headache | Before the intervention | 0.291±0.56 | 0.593±0.77 | 0.004 |
| One month later | 0.187±0.49 | 0.531±0.78 | 0.0001 | |
| Two month later | 0.167±0.46 | 0.479±0.69 | 0.001 | |
| 0.013 | 0.007 | |||
| Anorexia | Before the intervention | 0.708±0.76 | 0.479±0.66 | 0.031 |
| One month later | 0.583±0.73 | 0.333±0.6 | 0.01 | |
| Two month later | 0.562±0.72 | 0.364±0.58 | 0.059 | |
| 0.187 | 0.01 | |||
| Fainting | Before the intervention | 0.239±0.55 | 0.291±0.7 | 0.879 |
| One month later | 0.25±0.64 | 0.26±0.6 | 0.723 | |
| Two month later | 0.22±0.53 | 0.229±0.58 | 0.652 | |
| 0.962 | 0.05 | |||
| Fatigue | Before the intervention | 1.5±0.84 | 1.447±0.99 | 0.881 |
| One month later | 1.145±0.84 | 1.312±0.97 | 0.247 | |
| Two month later | 1.08±0.84 | 1.197±0.87 | 0.426 | |
| 0.001 | 0.006 | |||
| Mood changes | Before the intervention | 1.218±0.93 | 1.562±1.06 | 0.029 |
| One month later | 0.93±0.91 | 1.43±1.05 | 0.001 | |
| Two month later | 1.02±0.94 | 1.28±0.94 | 0.067 | |
| 0.001 | 0.001 | |||
| The total score of associated symptoms | Before the intervention | 7.22±3.98 | 7.62±3.97 | 0.743 |
| One month later | 5.41±3.07 | 6.18±3.8 | 0.262 | |
| Two month later | 4.93±3.54 | 5.62±3.54 | 0.131 | |
| 0.001 | 0.001 |
In general, the reported side effects of mefenamic acid group included gastrointestinal complications (n=9), and the complaint reported from chamomile group was bitter taste and vomiting (n=5).
4. DISCUSSION
The present study indicated that chamomile and mefenamic acid both decreased pain intensity, duration of pain and bleeding to the same degree, and chamomile seemed to be more effective in alleviating the symptoms related with dysmenorrhea.
The researchers showed that α-Bisabolol and its oxides in flowers of chamomile have therapeutic value. Different antispasmodic, anti-inflammatory, antihistamine, antioxidant, and anti-anxiety properties have been demonstrated for chamomile. Chamomile can relieve the pain during menstruation and prevent premature delivery due to the presence of flavonoids and alkaloids that have direct effect in the reduction of prostaglandins, as well as active ingredients such as spiroether (a strong antispasmodic agent) and having the ability to block calcium channels. On the other hand, chamomile is likely to suppress pain in central nervous system through phytoestrogenic effect, as well as Matrisin, flavonoids, Metoxicomarin, and Apigenin on central nervous system [43-48].
Studies have reported the reduction in amount of bleeding by chamomile, which is similar to other drugs such as mefenamic acid, ginger and fennel, and the decrease of bleeding quantity showed no significant difference between chamomile and the mentioned drugs [25, 49]; however, no information is available on the mechanism of chamomile action in this regard [50].
Increasing production of uterine prostaglandins is among the most likely factors causing severe menstrual bleeding . PGE2 and PGF2α increase blood flow to the uterus whereas E2 prostaglandins are vasodilators; therefore, these agents are associated with the risk of menstrual bleeding. Several researchers have shown that the inhibition of prostaglandin synthesis can reduce the quantity of menstrual bleeding in women. Chamomile inhibits cyclooxygenase and stops the production of prostaglandins and leukotrienes because of its anti-prostaglandin properties [28, 37].
According to traditional medicine principles, dysmenorrhea occurs due to the cold humor and the concentration of substances in the uterus, which requires a drug that is of warm humor capable of relieving the cold as well as eliminating the concentration of substances [51, 52]. Chamomile has a warm and dry temperament and can relieve uterine coldness; moreover, it is water soluble and can reduce concentration to help improve dysmenorrhea and its associated bleeding [53]. Karimian et al. conducted a clinical trial on 90 female students with primary dysmenorrhea. In this research, one group received 250 mg chamomile capsules and the other took 250 mg mefenamic acid capsules every 8 hours from 48 hours before menstruation to 24 hours after it, which was administered in two cycles.The results showed that chamomile was as effective as mefenamic acid in relieving dysmenorrhea and that the quantity of bleeding decreased in the two groups [39]. Study of Radfard showed that like yarrow, chamomile reduces the intensity and duration of pain during menstruation [43], and in a research performed in Tarbiat Modarres University, the intensity of pain and mean hemorrhage score decreased with chamomile that considerably mitigated pain [34].
Results of Najafi's study that compared bleeding rates between chamomile capsules and placebo using Higham chart indicated that the average bleeding decreased in the chamomile group [37]. Abedian showed that the bleeding quantity was decreased in both groups of chamomile and mefenamic acid [50]. Because chamomile is more effective in relieving dysmenorrhea if used before pain begins [25], the results of the present study that used chamomile as a sachet before the onset of menstruation are consistent with other studies using capsule, drop, tea, and different amounts of chamomile [22, 25, 54]. In a study conducted to compare the impact of chamomile extract and mefenamic acid on PMS, Sharifi reported that chamomile increased bleeding probably due to the type and concentration of active ingredients in chamomile in different cultivation sites and seasons of the year, which requires further investigations.
Examination of changes in symptoms related with primary dysmenorrhea in this research showed that mean severity of signs such as back pain, dizziness, diarrhea, headache, and fatigue was further decreased in chamomile group and that the difference was significant in relation to the headache symptom. Studies show that in addition to having antispasmodic and anti-inflammatory properties, chamomile is a stimulant factor of central nervous system. Apigenin, glycine, luteolin and flavonoids contained in chamomile bind benzodiazepine receptors, exert anxiolytic and sedative effects and are effective for stress and anxiety relief [43, 44, 55, 56]. It appears that chamomile can be used to treat nightmares as well as other sleep disorders [56]. Dysmenorrhea is associated with neurological symptoms, and it is predicted that the consumption of chamomile can improve the quality of sleep and reduce fatigue.
In addition, given the sedative effects of chamomile, the mental state as well as mood disorders such as irritability is likely to improve in those taking chamomile [57-59]. In the study of Janabi on primary dysmenorrhea, menstrual pain, anxiety and emotions of the chamomile tea group were significantly different with the control group [25]. Yazdani showed that the consumption of fennel and chamomile had a remarkable effect on dysmenorrhea as well as on three symptoms of premenstrual syndrome, namely abdominal and pelvic pain, fatigue and lethargy, depression together with anger. Chamomile has proven effective in relieving abdominal and pelvic pain, depression, anger, and fennel is more effective in reducing fatigue and lethargy [54]. Dadfar showed that chamomile extract can reduce the severity of PMS anxiety, depressive and retention symptoms; however, it had no effect on physical and emotional symptoms [55]. In another study, the physical symptoms were decreased to the same extent by chamomile extract and mefenamic acid, and the decrease of psychological symptoms by chamomile was more noticeable than mefenamic acid. It was concluded that flavonoids, apigenin and luteolin present in chamomile have anti-anxiety and sedative properties, while mefenamic acid had anti-prostaglandin effects [40]. As previously mentioned, the reason for the difference in results could be the variety of drug preparation methods affecting the active ingredients. In this research, there were gastrointestinal complications in subjects taking mefenamic acid, and while other studies show improvement of gastrointestinal complications using chamomile, it caused vomiting symptoms in our study. It appears that the bitter taste of chamomile sachet leads to low digestive tolerance and vomiting in these individuals. Researchers recommend the combination of chamomile and honey for treatment of dysmenorrhea, and further studies with long-term trials and a higher number of participants are necessary to ensure the definitive efficacy of herbal remedies.
CONCLUSION
The results of our study showed that chamomile sachet was effective in reducing pain as well as symptoms associated with primary dysmenorrhea and menstrual bleeding, which was comparable to mefenamic acid capsules. It is suggested that midwives and reproductive health professionals use complementary methods in clinical fields and address them as treatment options tailored to the needs of women.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved with code No. 2611 and ethics code ARAKMU.REC.1395.164 as well as IRCT code No. 2016100825031N5 at Arak University of Medical Sciences.
HUMAN AND ANIMAL RIGHTS
No animals were used for studies that are the basis of this research. All the human procedures were conducted in accordance with principles of Helsinki Declaration.
CONSENT FOR PUBLICATION
Written and informed consent has been obtained from all the students.
STANDARDS OF REPORTING
STROBE guidelines were followed.
AVAILABILITY OF DATA AND MATERIALS
The source of data and materials will not be shared because the legal owner of this research project is Arak University of Medical Sciences and does not permit data sharing.
FUNDING
This study was funded with the proposal approval code No. 2611 at Arak University of Medical Sciences in Iran.
CONFLICT OF INTEREST
Dr. Ali Bagheri is the Editorial Board Member of the The Open Civil Engineering Journal.
ACKNOWLEDGEMENTS
Declared none.


