All published articles of this journal are available on ScienceDirect.
Correlation between Medications Used during COVID Infection and Post-conditions after the Acute Phase of Infection: A Cross-sectional Study
Abstract
Background:
During the COVID-19 pandemic, off-label medication prescribing and utilizing herbal products and multiple vitamins in the treatment, prevention, and symptom management of COVID-19 was an urgently needed practice to halt the SARS-CoV-2 infection crisis and progression.
Objectives:
This study aimed to determine the correlation between medications used during the pandemic and SARS-CoV-2 infection post-recovery symptoms.
Methods:
A cross-sectional questionnaire-based study was conducted on recovered COVID-19 patients. There were 20 multiple-choice questions, including patient demographics, treatment, and post-recovery symptoms. Chi-square and Fisher’s exact tests were used to investigate significant relationships. In addition, Binary logistic regression was performed to determine confounders. Data were analyzed using SPSS version 22.
Results:
Medications and supplements varied in their therapeutic effects on SARS-CoV-2 post-recovery symptoms. Patients who took vitamin D and calcium experienced increased symptom frequency, and patients taking ACE inhibitors experienced more headaches and coughs. Furthermore, patients receiving azithromycin were asymptomatic after recovery. Patients who took H2 antagonists reported persistent headaches and muscle pain.
Conclusion:
Patients infected with SARS-CoV-2 have responded differently to medications, multivitamins, and herbal supplements. Patients reported resolution of some symptoms and persistence of others post-recovery. Therefore, expert opinion should be considered in COVID-19 management until randomized controlled trials answer many questions and determine medications' safety and efficacy in prevention, treatment strategies, and symptoms of SARS-CoV-2 infection post-recovery.
1. INTRODUCTION
COVID-19 was first reported in late December, 2019 and has since spread worldwide. It causes mild, moderate, or severe symptoms, such as fever, cough, fatigue, loss of smell and taste, shortness of breath, headache, chest pain, and anxiety
[1, 2]. Long-term COVID-19 is a condition in which recovered patients experience symptoms after recovery [3]. Owing to the limitations of therapeutic options and definite treatment for the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus, off-label use of many medications has been utilized during the pandemic for infection prophylaxis, treatment, or symptom alleviation, including paracetamol, nonsteroidal anti-inflammatory drugs, azithromycin, calcium, vitamin D, vitamin C, and zinc [4, 5]. In theory, the proposed mechanisms of action for certain medications place them as therapeutic options in targeting the pathophysiological processes of the virus life cycle by interfering with viral entry or attachment to the host cell or by enhancing the immune system response to overcome viral infection. Other agents also have an anti-inflammatory activity that limits the release of inflammatory mediators, causing symptoms and injury to organs in the human body.
The effectiveness of medication in COVID-19 disease post-recovery symptoms, presence, and duration shows an apparent discrepancy in the literature. For example, azithromycin, a macrolide antibiotic with a good safety profile and antiviral, anti-inflammatory, and immunomodulatory activities, has shown some benefits in SARS-CoV-2 infection. However, a randomized clinical trial suggested that oral azithromycin did not affect COVID-19 symptoms [6, 7]. Multiple vitamins, zinc, vitamin C, vitamin D, and calcium, have traditionally been used as immune boosters to reduce symptom severity of the common cold and flu and improve the overall health status. Zinc has in vitro activity against SARS-CoV-2; however, in a randomized clinical trial, zinc gluconate 50 mg daily, alone or in combination with vitamin C, did not reduce symptom duration in outpatients [8, 9]. In addition, a recent study revealed that severe COVID-19 infection was associated with low calcium levels, while vitamin D levels did not correlate with the severity of the infection [10].
Patients with chronic diseases or conditions lasting more than 3 months are more likely to experience severe COVID-19 [11, 12]. Medication for chronic disease management, including angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), statins, metformin, antacids, and histamine H2-receptor antagonists, may have positive or negative outcomes in COVID-19 patients [13]. Theoretically, ACE or ARBs may up-regulate the ACE2 receptor, facilitating viral entry into cells. SARS-CoV-2 virus utilizes the ACE2 receptor for host cell entry; however, blocking angiotensin in the respiratory system could reduce lung injury. Statins have anti-inflammatory activity that might benefit COVID-19 patients. Simultaneously, they might increase the expression of ACE receptors, increasing the risk of viral attachment and entry. Statins have been suggested to be associated with lower mortality risk [14-16]. Histamine H2-receptor antagonists are medications indicated for gastrointestinal oesophageal reflux disease with immunomodulatory effects and anti-inflammatory properties [17], and famotidine has been reported in some studies to improve patient COVID-19 outcomes [18, 19]. Many studies have also reported that metformin, an anti-diabetic drug, reduces the severity and mortality of the infection [20-22]
This study aimed to evaluate the association between different medications used during the COVID-19 pandemic and patients' health status of post-recovery symptoms. Furthermore, it analyzed the findings to offer information on the medication effects on post-COVID-19 patients, symptoms prevention, and certain chronic disease precautions.
2. MATERIALS AND METHODS
2.1. Study Design and Sample
This observational, questionnaire-based, cross-sectional study was conducted in Palestine between December, 2020 and February, 2021. The study participants were adults over 18 years of age who had previously been diagnosed with COVID-19 and recovered from the infection. People could participate by completing an online questionnaire or attending in-person interviews. Google Forms (Google Inc., USA) was used to design and disseminate the online questionnaires.
The representative sample size was calculated using the Raosoft sample size calculator website [23]. The calculated sample size was 382, based on the total number of cases diagnosed in Palestine by November 29th, 2020, and the total number of patients recovered from Covid-19 in the West Bank with a 95% confidence level and 5% accepted margin of error. This study included 740 recovered patients. Seventy-four participants were excluded because of duplication, incomplete responses, being under 18, or being asymptomatic during the infection.
The IRB committee of Birzeit University approved the study with reference number BZU-PNH-2006, and the research followed the guidelines of the Declarations of Helsinki.
2.2. Study Tool
The questionnaire was developed based on previously published research that represented a variety of post-COVID-19 symptoms and their correlation with chronic diseases, age, gender, and used medications, including antibiotics, pain killers, chronic disease medication for comorbid patients, and different types of vitamins used for treatment or as prophylaxis substances [24, 25].
The questionnaire was reviewed and modified based on feedback received from five multidisciplinary experts from the Faculty of Pharmacy, Nursing, and Health Professions. Furthermore, it was translated into Arabic as the primary language of the participants. Finally, a pilot study was conducted with 22 participants who recovered from the virus to examine the questionnaire's feasibility, validity, and clarity.
The questionnaire included 20 multiple-choice questions comprising three sections, including demographics, post-recovery symptoms, and medication usage. The first section has seven demographic questions regarding age, gender, place of residence, and health. In the second section, four questions addressed the types of symptoms experienced during infection and post-recovery. The answers were based on a yes/no basis. Finally, section three included nine questions exploring the medication administered during infection. Medications were presented in the questionnaire as pictures. The questionnaire is outlined in the supplementary material.
2.3. Statistical Analysis
All questions were coded and imported into IBM SPSS Statistics 22 for analysis. Descriptive statistics were used to present the data. First, age was classified into five categories (18-20, 21-30, 31-40, 41-50, and > 50 years. Next, multiple-choice questions were separated and entered as yes/no questions. Then, a Chi-square or Fisher’s exact test with a 95% confidence interval was used to evaluate relationships between demographics, post-infection symptoms, and medications. Finally, binary logistic regression was performed to determine confounders. A p-value of less than 0.05 represents a statistically significant relationship.
3. RESULTS
3.1. Demographic Information
A total of 666 participants were included in this study. Among them, 301 (45.2%) were between 21-30 years, with a mean age of 28.04 ± 11.642. About two-thirds of the patients were females; 458 (68.8%) and 95 (14.3%) had a chronic disease. Table 1 presents the demographic information of 666 respondents who completed the survey.
3.2. Medication Information
The majority of participants, 617 (92.6%), took vitamins or herbal supplements during the COVID-19 pandemic. A high percentage of participants used either vitamin C or zinc, 495 (74.3%) and 409 (61.4%), respectively, while more than half of the participants used both supplements, 375 (56.3%), and multivitamins were taken by 76 (11.4%). Furthermore, a considerable proportion of participants, 478 (71.8%), used herbal supplements during the COVID-19 infection. (Table 2).
| Variable | Categorization | Count (Frequencies) | Percentage % |
|---|---|---|---|
| Age | 18-20 | 185 | 27.8 |
| 21-30 | 301 | 45.2 | |
| 31-40 | 60 | 9 | |
| 41-49 | 80 | 12 | |
| >50 | 40 | 6 | |
| Sex | Male | 208 | 31.2 |
| Female | 458 | 68.8 | |
| Chronic diseases found | Blood Pressure | 40 | 6 |
| Diabetes | 24 | 3.6 | |
| Heart diseases and atherosclerosis | 13 | 2 | |
| Cholesterol | 16 | 2.4 | |
| Time since recovery | Less than a week | 190 | 28.5 |
| From a week to a month | 243 | 36.5 | |
| From a month to three months | 138 | 20.7 | |
| From three months to six months | 88 | 13.2 | |
| More than six months | 7 | 1.1 |
| Variable | Count (Frequencies) | Percentage % | |
|---|---|---|---|
| Used vitamins or supplements, or herbs during infection | Zinc | 409 | 61.4 |
| Vitamin C | 495 | 74.3 | |
| Vitamin D | 277 | 41.6 | |
| Calcium | 112 | 16.8 | |
| Herbs | 478 | 71.8 | |
| Multivitamin | 76 | 11.4 | |
| Vitamin C and Zinc | 375 | 56.3 | |
| Other remedies during infection | Gargling with salt and water | 111 | 16.7 |
| Nebulizing with herbs, medication, or others | 167 | 25.1 | |
| Pain killer | Paracetamol | 451 | 67.7 |
| Ibuprofen | 98 | 14.7 | |
| Antibiotics | Azithromycin | 191 | 28.7 |
| Amoxicillin | 56 | 8.4 | |
| Medications for chronic diseases during | Metformin | 28 | 4.2 |
| ACE Inhibitors | 37 | 5.6 | |
| Cholesterol Medications (STATINS) | 13 | 2 | |
| Acid reducer medication | Antacids | 34 | 5.1 |
| H2 inhibitors | 22 | 3.3 | |
| Prophylaxis treatment | Vitamin C | 114 | 17.1 |
| Vitamin D | 61 | 9.2 | |
| Zinc | 55 | 8.3 | |
| Multivitamins | 25 | 3.8 |
Regarding medications, 497 (74.6%) participants reported taking painkillers during infection. In addition, 239 (35.9%) took antibiotics, and 113 (17%) were administered medications for different chronic diseases.
3.3. Symptoms after Recovery
The participants were divided into two categories based on their reported symptoms. Category one included participants who experienced symptoms after recovery, while category two included participants with no post-recovery symptoms. Furthermore, the categorization included multiple variables, such as gender, age, chronic diseases, and medication utilization (Table 2). The results revealed that a higher percentage of females, 360 (78.6%), experienced symptoms after recovery than males, 98 (21.4%), with a p-value of < 0.001. A higher percentage of participants, 291 (77.6%), who took vitamin C and zinc during infection, experienced symptoms after recovery than participants who did not, i.e., 200 (68.7%), with a p-value of 0.01. Participants who took azithromycin, i.e., 157 (82.2%), experienced fewer post-recovery symptoms than those who did not take azithromycin, i.e., 334 (70.3%), with a p-value of 0.02. Moreover, participants who were not on medication were significantly less likely to complain of symptoms after recovery than others (p-value <0.001, 83 (61.5%)) (Table 3). Binary logistic regression results revealed no significant association between the administered medication (drugs) for patients with chronic disease and the persistence of symptoms after recovery. Another model entered the significant variables that revealed that being male, administrated azithromycin, not being administered any medication, or administered medication randomly made participants at lower risk of complaining of symptoms persistence after recovery (p-value <0.001, p-value=0.013, p-value=0.026, p- value= 0.005, respectively).
| Experienced Symptoms after Recovery | Did not Experience Symptoms after Recovery | p-value | |||
|---|---|---|---|---|---|
| Gender | Female | 360 (78.6) | 98 (21.4) | <0.001 | |
| Male | 131 (63) | 77 (37) | |||
| Age (years) | 18-20 | 130 (70.3) | 55 (29.7) | 0.588 | |
| 21-30 | 223 (74.1) | 78 (25.9) | |||
| 31-40 | 44 (73.3) | 16 (26.7) | |||
| 41-50 | 64 (80) | 16 (20) | |||
| > 50 | 30 (75) | 10 (25) | |||
| Chronic disease | Yes | 73 (76.8) | 22 (23.2) | 0.456 | |
| No | 418 (73.2) | 153 (26.8) | |||
| Vitamins administered during infection | Vit. C | Yes | 374 (75.6) | 121 (24.4) | 0.068 |
| No | 117 (68.4) | 54 (31.6) | |||
| Vit. D | Yes | 214 (77.3) | 63 (22.7) | 0.08 | |
| No | 277 (71.2) | 112 (28.8) | |||
| Zn | Yes | 311 (76) | 98 (24) | 0.087 | |
| No | 180 (70) | 77 (30) | |||
| Ca | Yes | 89 (79.5) | 23 (20.5) | 0.13 | |
| No | 402 (72.6) | 152 (27.4) | |||
| Combination Vit. C and Zn |
Yes | 291 (77.6) | 84 (22.4) | 0.01 | |
| No | 200 (68.7) | 91 (31.3) | |||
| Multivitamin | Yes | 61 (80.3) | 15 (19.7) | 0.169 | |
| No | 430 (72.9) | 160 (27.1) | |||
| Vitamins administered as prophylaxis | Vit. D | Yes | 51 (83.6) | 10 (16.4) | 0.066 |
| No | 440 (72.7) | 51 (83.6) | |||
| Combination Vit. C and Zn |
Yes | 33 (78.6) | 9 (21.4) | 0.089 | |
| No | 458 (73.4) | 166 (26.6) | |||
| Multivitamin | Yes | 21 (84) | 4 (16) | 0.234 | |
| No | 470 (73.3) | 171 (26.7) | |||
| Azithromycin | Yes | 157 (82.2) | 34 (17.8) | 0.002 | |
| No | 334 (70.3) | 141 (29.7) | |||
| Blood pressure medications (ACE inhibitors) | Yes | 32 (86.5) | 5 (13.5) | 0.07 | |
| No | 459 (73) | 170 (27) | |||
| Diabetes medications (Metformin) |
Yes | 22 (78.6) | 6 (21.4) | 0.552 | |
| No | 469 (73.5) | 169 (26.5) | |||
| Antacids | Yes | 29 (85.3) | 5 (14.7) | 0.116 | |
| No | 462 (73.1) | 170 (26.9) | |||
| Gastrointestinal medications (H2 receptor blockers) | Yes | 20 (90.9) | 2 (9.1) | 0.063 | |
| No | 471 (73.1) | 173 (26.9) | |||
| Cholesterol medications (statins) | Yes | 12 (92.3) | 1 (7.7) | 0.201 | |
| No | 479 (73.4) | 174 (26.6) | |||
| Amoxicillin | Yes | 43 (76.8) | 13 (23.2) | 0.586 | |
| No | 448 (73.4) | 162 (26.6) | |||
| Medication commitment | Full | 225 (77.9) | 64 (22.1) | < 0.001 | |
| Partial | 91 (82) | 20 (18) | |||
| Randomly | 92 (70.2) | 39 (29.8) | |||
| No medication | 83 (61.5) | 52 (38.5) | |||
3.4. Correlations between Medications and Symptoms after Recovery
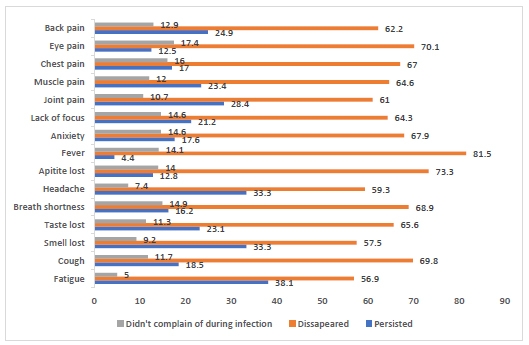
COVID-19 is associated with various symptoms. As shown in Fig. (1), the most commonly reported symptoms were fever and fatigue, followed by headache (95.6%, 95%, and 92.6%, respectively). Many symptoms did not disappear after the participants recovered from the infection, such as fatigue, loss of smell, and loss of taste (38.1%, 33.3%, and 33.3%, respectively), while a minority complained of persistent fever (4.4%).
Participants who took azithromycin during infection were more likely to complain of persistent symptoms, including 45 (27.1%) who experienced continued chest pain, with a p-value of 0.008, and 90 (48.1%) who suffered from fatigue after recovery with a p-value of 0.008. Furthermore, 51 (30.7%) endured lacked focus, with a p-value of 0.035.
In participants who took ACE inhibitors during infection, 14 (43.8%) had cough continued after recovery with a p-value of 0.001, 18 (54.5%) suffered from headache after recovery with a p-value of 0.022, and 18 (54.5%) endured continued muscle pain, 11 (34.4%) chest pain, 14 (46.7%) back pain, and 16 (50) joint pain with a p-value< 0.001, p-value= 0.04, p-value=0.025, p-value = 0.023, respectively.
Participants who took H2 antagonists reported a lack of focus, headache, muscle pain, and fatigue (p-value = 0.014, p-value = 0.04, p-value =0.011, and p-value =0.022, respectively). In addition, participants taking calcium and vitamin D supplements reported several post-recovery symptoms, including lack of focus, joint pain, muscle pain, loss of appetite, anxiety, and persistent fatigue, with a p-value of <0.05 (Table 4).
| Drug | Symptom | Total | Answer | Medication | p-value | |
|---|---|---|---|---|---|---|
| Yes | No | |||||
| Participants took azithromycin | Chest pain | 559 | Yes | 45 (27.1) | 68 (17.3) | 0.008 |
| No | 121 (72.9) | 325 (82.7) | ||||
| Fatigue | 633 | Yes | 90 (48.1) | 164 (36.8) | 0.008 | |
| No | 97 (51.9) | 282 (63.2) | ||||
| Lack of focus | 569 | Yes | 51 (30.7) | 90 (22.3) | 0.035 | |
| No | 115 (69.3) | 313 (77.7) | ||||
| Participants took blood pressure medications | Cough | 588 | Yes | 14 (43.8) | 109 (19.6) | 0.001 |
| No | 18 (56.3) | 447 (80.4) | ||||
| Headache | 617 | Yes | 18 (54.5) | 204 (34.9) | 0.022 | |
| No | 15 (45.5) | 380 (65.1) | ||||
| Muscle pain | 586 | Yes | 18 (54.5)) | 138 (25) | <0.001 | |
| No | 15 (45.5) | 415 (75) | ||||
| Chest pain | 559 | Yes | 11 (34.4) | 102 (19.4) | 0.04 | |
| No | 21 (65.5) | 425 (80.6) | ||||
| Back pain | 580 | Yes | 14 (46.7) | 152 (27.6) | 0.025 | |
| No | 16 (53.3) | 398 (72.4) | ||||
| Joint pain | 595 | Yes | 16 (50) | 173 (30.7) | 0.023 | |
| No | 16 (50) | 390 (69.3) | ||||
| Participants took H2 antagonists | Lack of focus | 569 | Yes | 10 (47.6) | 131 (23.9) | 0.014 |
| No | 11 (52.4) | 417 (76.1) | ||||
| Headache | 617 | Yes | 12 (57.1) | 210 (35.2) | 0.04 | |
| No | 9 (42.9) | 386 (64.8) | ||||
| Muscle pain | 586 | Yes | 11 (50.0) | 145 (25.7) | 0.011 | |
| No | 11 (50.0) | 419 (74.3) | ||||
| Fatigue | 633 | Yes | 14 (63.6) | 240 (39.3) | 0.022 | |
| No | 8 (36.4) | 371 (60.7) | ||||
| Participants took calcium | Lack of focus | 569 | Yes | 36 (36) | 105 (22.4) | 0.004 |
| No | 64 (64) | 364 (77.6) | ||||
| Joint pain | 595 | Yes | 45 (43.7) | 144 (29.3) | 0.004 | |
| No | 58 (56.3) | 348 (70.7) | ||||
| Muscle pain | 586 | Yes | 39 (38.6) | 117 (24.1) | 0.003 | |
| No | 62 (61.4) | 368 (75.9) | ||||
| Loss of appetite | 573 | Yes | 23 (22.8) | 62 (13.1) | 0.013 | |
| No | 78 (77.2) | 410 (86.9) | ||||
| Anxiety and tension | 569 | Yes | 32 (32) | 85 (18.1) | 0.002 | |
| No | 68 (68) | 384 (81.9) | ||||
| Fatigue | 633 | Yes | 53 (49.1) | 201 (38.3) | 0.037 | |
| No | 55 (50.9) | 324 (61.7) | ||||
| Participants took vitamin D | Muscle pain | 586 | Yes | 76 (31.4) | 80 (23.3) | 0.028 |
| No | 166 (68.6) | 264 (76.7) | ||||
| Joint pain | 595 | Yes | 90 (36.9) | 99 (28.2) | 0.025 | |
| No | 154 (63.1) | 252 (71.8) | ||||
| Fatigue | 633 | Yes | 121 (46.2) | 133 (35.8) | 0.009 | |
| No | 141 (53.8) | 238 (64.2) | ||||
4. DISCUSSION
During the COVID-19 pandemic, the limited availability of data created a challenging clinical decision for the healthcare society to adopt therapeutic protocols based on scientific evidence to recommend promising medications to treat, prevent, or manage COVID-19 patients. For example, the MATH plus protocol for COVID-19 management developed by Dr. Paul Maric at the Eastern Virginia Medical School includes multiple proposed mechanisms of action and biological effects that are safe, cheap, and readily available. The protocol included vitamin C, vitamin D, zinc, thiamine, melatonin, famotidine, and statins [26]. However, clinical trials and more studies are needed to confirm their benefits in decreasing symptoms and reducing the mortality and morbidity of the disease. According to many studies conducted in different countries related to COVID-19, many patients continue to experience symptoms after infection recovery [25]. In addition, some medications and vitamins affect patients’ health during and post-infection [27, 28].
The majority of the study participants received multiple vitamins as a treatment or prophylaxis for COVID-19 symptoms. However, post-symptom resolutions and durations reported varied among participants based on gender and the type of medication they were taking.
The present research results revealed that females are more likely to experience symptoms after recovery than males [25]. The issue of gender as a potential risk factor for developing post-COVID symptoms has been previously reported in the literature [29, 30]. Nevertheless, other studies reported no difference associated with symptoms of COVID-19 among both genders [24, 31, 32].
The majority of participants who took vitamin C and zinc experienced at least one symptom after recovery, with a significant correlation compared to participants who did not take these vitamins. Meanwhile, studies have shown that vitamin C and zinc intake do not decrease the frequency of symptoms during infection [33, 34]. Multivitamins containing vitamins C and D and zinc have no significant relationship with symptoms after recovery: furthermore, they do not affect symptom severity [35]. Although some studies have recommended vitamin D and claimed that it reduces infection severity [36], our findings showed that it does not affect symptoms after recovery.
Calcium and vitamin D deficiencies affect many body systems and functions. For example, during the COVID-19 pandemic, vitamin D deficiency and low serum calcium levels were associated with poor prognosis and adverse outcomes [36]. In this study, participants who took calcium during the infection did not experience post-infection anxiety and tension. Moreover, calcium has been reported in some studies to improve anxiety and restlessness [37]. In addition, more than half of the participants who took calcium supplements did not continue to experience joint pain. Calcium and vitamin D improve bone, joint, and muscle health and reduce joint pain and inflammation [38]. Furthermore, muscle pain and lack of concentration did not persist in patients who took calcium during COVID-19; calcium deficiency may initiate or exacerbate these symptoms [39]. Furthermore, participants who were on vitamin D for prophylaxis did not endure persistent fatigue, muscle pain, back pain, joint pain, lack of focus, and loss of appetite, which are symptoms of a vitamin D deficiency [40-42], and these participants had high vitamin D storage in their bodies, implying that it could prevent the persistence or exacerbation of these symptoms.
Azithromycin, a commonly prescribed antibiotic for respiratory tract infections, is well tolerated and has a good safety profile. In the present study, most patients who received azithromycin experienced fewer symptoms after recovery, with a significant correlation. However, many studies have suggested that using azithromycin to treat COVID-19 is ineffective [43-45]. An observational study used azithromycin with hydroxychloroquine to prevent bacterial superinfection in COVID-19 patients. Although the study suggested that the combination can reduce viral load and improve the clinical course, the sample size of this study was too small and had no comparator group [46]. Furthermore, in a randomized controlled clinical trial that included hospitalized patients with mild to moderate COVID-19 symptoms, azithromycin alone or in combination with hydroxychloroquine did not improve clinical status in 15 days compared to the standard of care [47].
Most participants in the study who received ACE inhibitors, a medication used for a broad spectrum of cardiovascular disorders, experienced at least one significant symptom after recovery. ACE inhibitors might increase the expression of ACE 2 receptors, which aids viral entry into cells and leads to increased viral load [48]. More than half of the patients on ACE inhibitors did not experience cough or headache after recovery [49], even though the cough is a common side effect. One study suggested that ACE inhibitors were associated with a reduced risk of headache compared to other hypertensive drugs [50]. Furthermore, it is recommended that patients with chronic diseases on ACE inhibitors should not discontinue the medication due to its life-saving effects during COVID-19 infection [51].
Statins are another cardiovascular disease medication recommended by many clinical guidelines for the primary and secondary prevention of atherosclerotic cardiovascular disease. Statins are thought to have immunomodulatory and anti-inflammatory activities. In this study, patients who took statins did not experience continued symptoms after recovery compared to others. In other studies, statins showed an effect on taste loss due to alerting the action of taste buds when sending and receiving nerve signals and the amount of saliva and its content [52]. Furthermore, 69.2% of these participants did not endure a continued loss of appetite, which might have resulted from statins reducing leptin levels, thus decreasing the feeling of fullness [53, 54]. In a meta-analysis, statin therapy was associated with a 35% decrease in the adjusted mortality risk in hospitalized COVID-19 patients [55]. In another meta-analysis of 9,000 COVID-19 patients assessing the risk of severe COVID-19 disease or mortality in statin users versus non-users, the statin group was associated with a reduced risk of severe or fatal SARS-CoV-2 infection (HR 0.7, 95% CI 0.53 to 0.94). Statins can improve post-COVID-19 loss of appetite, and calcium can do so significantly, with an unknown mechanism.
Famotidine is a histamine blocker and is commonly used in gastrointestinal disorders. Subjects who took H2 receptor antagonists experienced post-covid symptoms, although studies have shown that these medications have a promising effect on COVID-19 [56]. Although more than half of the subjects had continued muscle pain and headache, these findings could be attributed to the side effects of the drug [57].
5. LIMITATIONS
The results of this study may not be extrapolated to the general population due to the small number of participants in subgroups: such as participants with chronic diseases. In addition, critically ill patients who were hospitalized at the time of the study were unable to complete the study. Furthermore, patients who died during the pandemic were not included in the study; therefore, survivor bias might have affected the results. Furthermore, participants who completed the questionnaire may have had a recall bias. In addition, a selection bias might have occurred due to voluntary participation. Finally, the results of chronic disease patients might be confounded due to the disease’s reversed effect on the patients’ health.
CONCLUSION
The treatment and prophylaxis for COVID-19 disease continue to evolve. In this study, azithromycin has different effects on post-recovery symptoms experienced by patients infected with SARS-CoV-2 and their health status. The results revealed that patients on ACE inhibitors experienced headaches, cough, and general fatigue after recovery. Patients on calcium and vitamin D did not report any recurrence of headaches, continued muscle and joint pain, anxiety and tension, and lack of focus. Patients who took H2 antagonists experienced persistent lack of focus, headaches, fatigue, and muscle pain. Multivitamins did not affect post-recovery symptoms. Expert opinion should be considered in COVID-19 management until randomized controlled trials answer questions and determine medication's role in prevention, treatment strategies, and symptoms of SARS-CoV-2 infection post-recovery.
LIST OF ABBREVIATIONS
| SARS-CoV-2 | = Severe Acute Respiratory Syndrome Coronavirus-2 |
| ACE inhibitors | = Angiotensin-Converting Enzyme inhibitors |
| ARBs | = Angiotensin Receptor Blockers |
| HR | = Hazard Ratio |
| CI | = Confidence Interval |
AUTHORS' CONTRIBUTION
TO, MJ, CM, ME, AD, and MQ wrote the first draft of the manuscript, collected data, and analyzed the data. H.N and ADA planned, coordinated, and supervised the work. N.SH supervised data analysis. AKR reviewed the final edition of the manuscript. All authors contributed to the review of the final edition of the manuscript, revised the manuscript, and approved the final version.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The IRB committee of Birzeit University approved the study with the reference number BZU-PNH-2006.
HUMAN AND ANIMAL RIGHTS
No animals were used in the studies that are the basis of this research. All the humans were used in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2013 (http://ethics.iit.edu/ecodes/node/3931).
CONSENT FOR PUBLICATION
Consent was obtained from all the participants of this study.
STANDARD OF REPORTING
STROBE Guideline were followed in this study.
AVAILABILITY OF DATA AND MATERIALS
The corresponding author [H.N.] will provide the data supporting the conclusions of this study.
FUNDING
None.
CONFLICTS OF INTEREST
There is no conflict of interest declared by the authors.
ACKNOWLEDGEMENTS
The authors would like to thank the professors of Faculty of Pharmacy, Nursing, and Health Professions of Birzeit University for their cooperation and all participants who participated in the survey.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the Publisher’s website.


